
Concept explainers
(a)
Interpretation:
Given Lewis structure is to be drawn using condensed formula.
Concept introduction:
The condensed formula indicates how the atoms should be connected in a given molecule. To arrive at a total charge of zero, each carbon should have a maximum of four bonds while each oxygen should have a maximum of two bonds and two lone pairs. A parenthesis in the condensed formula represents that the repetitive unit is attached to the previous carbon atom. The
Answer to Problem 1.61P
The condensed formula for the first Lewis structure is:
Explanation of Solution
The given Lewis structure is:
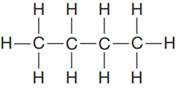
In the given Lewis structure, there are two
The condensed formula for the first Lewis structure is shown as:
(b)
Interpretation:
Given Lewis structure is to be drawn using condensed formula.
Concept introduction:
The condensed formula indicates how the atoms should be connected in a given molecule. To arrive at a total charge of zero, each carbon should have a maximum of four bonds while each oxygen should have a maximum of two bonds and two lone pairs. A parenthesis in the condensed formula represents that the repetitive unit is attached to the previous carbon atom. The
Answer to Problem 1.61P
The condensed structure for the given Lewis structure is:
Explanation of Solution
The given Lewis structure is:
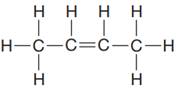
In the structure above, there are two
The condensed formula for the given Lewis structure is shown as:
(c)
Interpretation:
Given Lewis structure is to be drawn using condensed formula.
Concept introduction:
The condensed formula indicates how the atoms should be connected in a given molecule. To arrive at a total charge of zero, each carbon should have a maximum of four bonds while each oxygen should have a maximum of two bonds and two lone pairs. A parenthesis in the condensed formula represents that the repetitive unit is attached to the previous carbon atom. The
Answer to Problem 1.61P
The condensed structure for the given Lewis structure is:
Explanation of Solution
The given Lewis structure is:
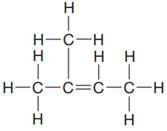
In the structure above, there are two
The condensed formula for the given Lewis structure is shown as:
(d)
Interpretation:
Given Lewis structure is to be drawn using condensed formula.
Concept introduction:
The condensed formula indicates how the atoms should be connected in a given molecule. To arrive at a total charge of zero, each carbon should have a maximum of four bonds while each oxygen should have a maximum of two bonds and two lone pairs. A parenthesis in the condensed formula represents that the repetitive unit is attached to the previous carbon atom. The
Answer to Problem 1.61P
The condensed structure for the given Lewis structure is:
Explanation of Solution
The given Lewis structure is:
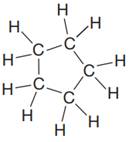
The structure above is a five carbon ring structure. Rings are generally now shown in their condensed formulas, but they are commonly shown in their partially condensed form. Thus, the condensed formula for the given Lewis structure is:
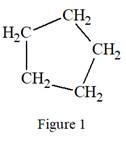
The condensed formula for the given Lewis structure is shown in Figure 1.
(e)
Interpretation:
Given Lewis structure is to be drawn using condensed formula.
Concept introduction:
The condensed formula indicates how the atoms should be connected in a given molecule. To arrive at a total charge of zero, each carbon should have a maximum of four bonds while each oxygen should have a maximum of two bonds and two lone pairs. A parenthesis in the condensed formula represents that the repetitive unit is attached to the previous carbon atom. The
Answer to Problem 1.61P
The condensed structure for the given Lewis structure is:
Explanation of Solution
The given Lewis structure is:
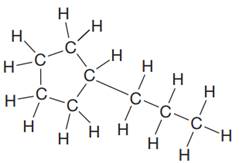
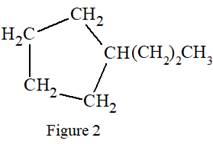
In the structure above, there is a five membered ring on the left side and a branch of three carbon atoms. Rings are generally now shown in their condensed formulas, but they are commonly shown in their partially condensed form. On one of the carbon atoms in the five membered ring, two
Thus, the condensed formula for the given Lewis structure is:

The condensed formula for the given Lewis structure is shown in Figure 2.
(f)
Interpretation:
Given Lewis structure is to be drawn using condensed formula.
Concept introduction:
The condensed formula indicates how the atoms should be connected in a given molecule. To arrive at a total charge of zero, each carbon should have a maximum of four bonds while each oxygen should have a maximum of two bonds and two lone pairs. A parenthesis in the condensed formula represents that the repetitive unit is attached to the previous carbon atom. The
Answer to Problem 1.61P
The condensed structure for the given Lewis structure is:
Explanation of Solution
The given Lewis structure is:
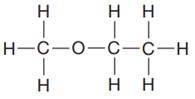
In the structure above, a
The condensed formula for the given Lewis structure is
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
- What is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forward
- Q Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forwardb. ὋΗ CH3CH2OH H2SO4arrow_forward
- For the reaction A (g) → 3 B (g), Kp = 0.379 at 298 K. What is the value of ∆G for this reaction at 298 K when the partial pressures of A and B are 5.70 atm and 0.250 atm?arrow_forward14. Calculate the concentrations of Ag+, Ag(S2O3), and Ag(S2O3)23- in a solution prepared by mixing 150.0 mL of 1.00×10-3 M AgNO3 with 200.0 mL of 5.00 M Na2S2O3 Ag+ + S20 Ag(S203)¯ K₁ = 7.4 × 108 Ag(S203)¯ + S20¯ = Ag(S203) K₂ = 3.9 x 104arrow_forwardΗΝ, cyclohexanone pH 4-5 Draw Enamine I I CH3CH2Br THF, reflux H3O+ I Drawing Draw Iminium Ionarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
