
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.58P
Predict the geometry around each highlighted atom.
a. 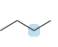 b.
b. 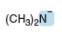 c.
c. 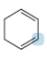 d.
d. 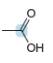 e.
e. 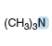
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the titration of a divalent metal ion (M2+) with EDTA, the stoichiometry of the reaction is typically:
1:1 (one mole of EDTA per mole of metal ion)
2:1 (two moles of EDTA per mole of metal ion)
1:2 (one mole of EDTA per two moles of metal ion)
None of the above
Please help me solve this reaction.
Indicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.
Chapter 1 Solutions
ORGANIC CHEMISTRY
Ch. 1 - While the most common isotope of nitrogen has a...Ch. 1 - Label each bond in the following compounds as...Ch. 1 - How many covalent bonds are predicted for each...Ch. 1 - Draw a valid Lewis structure for each species. a....Ch. 1 - Draw an acceptable Lewis structure for each...Ch. 1 - Prob. 1.6PCh. 1 - Draw a Lewis structure for each ion. a. CH3Ob....Ch. 1 - Prob. 1.8PCh. 1 - Draw Lewis structures for each molecular formula....Ch. 1 - Classify each pair of compounds as isomers or...
Ch. 1 - Prob. 1.11PCh. 1 - Prob. 1.12PCh. 1 - Prob. 1.13PCh. 1 - Draw a second resonance structure for each species...Ch. 1 - Prob. 1.15PCh. 1 - Prob. 1.16PCh. 1 - Prob. 1.17PCh. 1 - Prob. 1.18PCh. 1 - Using the principles of VSEPR theory, you can...Ch. 1 - Convert each condensed formula to a Lewis...Ch. 1 - Prob. 1.21PCh. 1 - Prob. 1.22PCh. 1 - Convert each skeletal structure to a complete...Ch. 1 - What is the molecular formula of quinine, the...Ch. 1 - Draw in all hydrogens and lone pairs on the...Ch. 1 - Prob. 1.26PCh. 1 - What orbitals are used to form each of the CC, and...Ch. 1 - What orbitals are used to form each bond in the...Ch. 1 - Determine the hybridization around the highlighted...Ch. 1 - The unmistakable odor of a freshly cut cucumber is...Ch. 1 - Prob. 1.31PCh. 1 - Rank the following atoms in order of increasing...Ch. 1 - Prob. 1.33PCh. 1 - Prob. 1.34PCh. 1 - Provide the following information about...Ch. 1 - Use the ball-and-stick model to answer each...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Assign formal charges to each carbon atom in the...Ch. 1 - Assign formal charges to each and atom in the...Ch. 1 - Prob. 1.41PCh. 1 - Prob. 1.42PCh. 1 - Prob. 1.43PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 1.46PCh. 1 - Prob. 1.47PCh. 1 - Prob. 1.48PCh. 1 - Prob. 1.49PCh. 1 - Prob. 1.50PCh. 1 - Prob. 1.51PCh. 1 - Prob. 1.52PCh. 1 - Consider compounds A-D, which contain both a...Ch. 1 - Prob. 1.54PCh. 1 - Prob. 1.55PCh. 1 - 1.56 Consider the compounds and ions with curved...Ch. 1 - 1.57 Predict all bond angles in each...Ch. 1 - 1.58 Predict the geometry around each highlighted...Ch. 1 - Prob. 1.59PCh. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - 1.61 Convert each molecule into a skeletal...Ch. 1 - Prob. 1.62PCh. 1 - Prob. 1.63PCh. 1 - Predict the hybridization and geometry around each...Ch. 1 - Prob. 1.65PCh. 1 - Ketene, , is an unusual organic molecule that has...Ch. 1 - Rank the following bonds in order of increasing...Ch. 1 - Prob. 1.68PCh. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Prob. 1.71PCh. 1 - Anacin is an over-the-counter pain reliever that...Ch. 1 - Answer the following questions about acetonitrile...Ch. 1 - Prob. 1.74PCh. 1 - 1.75 The principles of this chapter can be...Ch. 1 -
a. What is the hybridization of each N atom in...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 1.80PCh. 1 - Prob. 1.81PCh. 1 - Prob. 1.82PCh. 1 - Prob. 1.83PCh. 1 - Prob. 1.84PCh. 1 - Prob. 1.85P
Additional Science Textbook Solutions
Find more solutions based on key concepts
An electric motor has an effective resistance of 32.0 and an inductive reactance of 45.0 when working under l...
Fundamentals of Physics Extended
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
Some people consider Pasteur or Koch to be the Father of Microbiology, rather than Leeuwenhoek. Why might they ...
Microbiology with Diseases by Body System (5th Edition)
Why is it necessary to be in a pressurized cabin when flying at 30,000 feet?
Anatomy & Physiology (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY