
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.10P
Classify each pair of compounds as isomers or resonance structures.
a.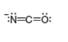 and
and 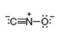 b.
b. 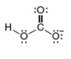 and
and 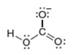
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Naming and drawing secondary
Write the systematic (IUPAC) name for each of the following organic molecules:
CH3
Z
structure
CH3
CH2
CH2
N-CH3
CH3-CH2-CH2-CH-CH3
NH
CH3-CH-CH2-CH2-CH2-CH2-CH2-CH3
Explanation
Check
☐
name
☐
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C
G
C
This question shows how molecular orbital (MO) theory can be used to understand the chemical
properties of elemental oxygen O₂ and its anionic derivative superoxide Oz.
a)
Draw the MO energy diagram for both O2 and O2. Clearly label your diagram with atomic orbital names
and molecular orbital symmetry labels and include electrons.
Draw the Lewis structure of O2. How does the MO description of O2 differ from the Lewis structure, and
how does this difference relate to the high reactivity and magnetic properties of oxygen?
) Use the MO diagram in (a) to explain the difference in bond length and bond energy between superoxide
ion (Oz, 135 pm, 360 kJ/mol) and oxygen (O2, 120.8 pm, 494 kJ/mol).
Please draw
Chapter 1 Solutions
ORGANIC CHEMISTRY
Ch. 1 - While the most common isotope of nitrogen has a...Ch. 1 - Label each bond in the following compounds as...Ch. 1 - How many covalent bonds are predicted for each...Ch. 1 - Draw a valid Lewis structure for each species. a....Ch. 1 - Draw an acceptable Lewis structure for each...Ch. 1 - Prob. 1.6PCh. 1 - Draw a Lewis structure for each ion. a. CH3Ob....Ch. 1 - Prob. 1.8PCh. 1 - Draw Lewis structures for each molecular formula....Ch. 1 - Classify each pair of compounds as isomers or...
Ch. 1 - Prob. 1.11PCh. 1 - Prob. 1.12PCh. 1 - Prob. 1.13PCh. 1 - Draw a second resonance structure for each species...Ch. 1 - Prob. 1.15PCh. 1 - Prob. 1.16PCh. 1 - Prob. 1.17PCh. 1 - Prob. 1.18PCh. 1 - Using the principles of VSEPR theory, you can...Ch. 1 - Convert each condensed formula to a Lewis...Ch. 1 - Prob. 1.21PCh. 1 - Prob. 1.22PCh. 1 - Convert each skeletal structure to a complete...Ch. 1 - What is the molecular formula of quinine, the...Ch. 1 - Draw in all hydrogens and lone pairs on the...Ch. 1 - Prob. 1.26PCh. 1 - What orbitals are used to form each of the CC, and...Ch. 1 - What orbitals are used to form each bond in the...Ch. 1 - Determine the hybridization around the highlighted...Ch. 1 - The unmistakable odor of a freshly cut cucumber is...Ch. 1 - Prob. 1.31PCh. 1 - Rank the following atoms in order of increasing...Ch. 1 - Prob. 1.33PCh. 1 - Prob. 1.34PCh. 1 - Provide the following information about...Ch. 1 - Use the ball-and-stick model to answer each...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Assign formal charges to each carbon atom in the...Ch. 1 - Assign formal charges to each and atom in the...Ch. 1 - Prob. 1.41PCh. 1 - Prob. 1.42PCh. 1 - Prob. 1.43PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 1.46PCh. 1 - Prob. 1.47PCh. 1 - Prob. 1.48PCh. 1 - Prob. 1.49PCh. 1 - Prob. 1.50PCh. 1 - Prob. 1.51PCh. 1 - Prob. 1.52PCh. 1 - Consider compounds A-D, which contain both a...Ch. 1 - Prob. 1.54PCh. 1 - Prob. 1.55PCh. 1 - 1.56 Consider the compounds and ions with curved...Ch. 1 - 1.57 Predict all bond angles in each...Ch. 1 - 1.58 Predict the geometry around each highlighted...Ch. 1 - Prob. 1.59PCh. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - 1.61 Convert each molecule into a skeletal...Ch. 1 - Prob. 1.62PCh. 1 - Prob. 1.63PCh. 1 - Predict the hybridization and geometry around each...Ch. 1 - Prob. 1.65PCh. 1 - Ketene, , is an unusual organic molecule that has...Ch. 1 - Rank the following bonds in order of increasing...Ch. 1 - Prob. 1.68PCh. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Prob. 1.71PCh. 1 - Anacin is an over-the-counter pain reliever that...Ch. 1 - Answer the following questions about acetonitrile...Ch. 1 - Prob. 1.74PCh. 1 - 1.75 The principles of this chapter can be...Ch. 1 -
a. What is the hybridization of each N atom in...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 1.80PCh. 1 - Prob. 1.81PCh. 1 - Prob. 1.82PCh. 1 - Prob. 1.83PCh. 1 - Prob. 1.84PCh. 1 - Prob. 1.85P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- -Page: 8 nsition metal ions have high-spin aqua complexes except one: [Co(HO)₁]". What is the d-configuration, oxidation state of the metal in [Co(H:O))"? Name and draw the geometry of [Co(H2O)]? b) Draw energy diagrams showing the splitting of the five d orbitals of Co for the two possible electron configurations of [Co(H2O)]: Knowing that A = 16 750 cm and Пl. = 21 000 cm, calculate the configuration energy (.e., balance or ligand-field stabilization energy and pairing energy) for both low spin and high spin configurations of [Co(H2O)]. Which configuration seems more stable at this point of the analysis? (Note that 349.76 cm = 1 kJ/mol) Exchange energy (IT) was not taken into account in part (d), but it plays a role. Assuming exchange an occur within t29 and within eg (but not between tz, and ea), how many exchanges are possible in the low in configuration vs in the high spin configuration? What can you say about the importance of exchange energy 07arrow_forwardDraw everything please on a piece of paper explaining each steparrow_forwardDefine crystalline, polycrystalline and amorphous materials What crystal system and Bravais lattices are shown in the figure immediately below? What do a, b, C, a, ẞ and y represent and what are their values? You can label the Bravais lattices directly above or under the figure. C aarrow_forward
- 32. The diagrams below show the band structure of an intrinsic semiconductor at absolute zero and room temperature. Room Temperature EF E OK Ep- a) In the space below, sketch a similar pair of diagrams for an n-type semiconductor. D) Give the definition and an example of (i) an intrinsic semiconductor and (ii) an n-type semiconductor.arrow_forward29. a) i Which energy diagram best represents the d-electrons in tetrahedral [Co(NH3)4]²+? b) ii c) iii d) iv 11 ་ ↑↓ ↑t t ↑↓ ↑↓ e) none of these ii In1 According to Slater's rules, what is the effective nuclear charge experienced by a 3d electron in 30. Ge? a) 32.00 b) 21.15 c) 16.05 d) 14.00 e) 10.85arrow_forwardRegarding Lowis structuros and geometrios, Draw Lewis structures for the following: SOF4, SO, ICI, XeO2F4, SeF and XeO3. For each one, indicate the observed molecular geometry it adopts.arrow_forward
- Explain the following statements with equations: - The fusion product of an organic compund with sodium metal is an alkaline solution - The test for elements should be done before the solubility tests. - Using less sodium than the organic compound in the ignition test might cause a problem to detect the presence of the nitrogen and sulfur - Formation of colored product when adding ferric acid chloride to phenol solutionarrow_forward31 Indicate the symbol, mass number and the atomic number of the missing product in each of the following nuclear reactions. a) 13/3 N 41 b) 11 Ca 20 c) 90 38 Sr → 133 C + ? + - 6 0 e →? 90 Y + ? 39 11 d) 22 Na → ? + + 1 B +1 β Toarrow_forwardPlease drawarrow_forward
- 9. compore the Following two Venctions IN termy Of Ronction Rate and explan in detail the reasoning that led to your conclusion +He p₁₂ 11- ㅐ 15 .. +He H #H H / H b. Compare the Following too reactions 14 terms of reaction Rate and explain in detail the reasoning that led to your conclusion Н d-C- tłu Na +2446 е -ll +2n "Harrow_forwarda. •Write all of the possible products For the Following ronction А ----- H - H H + H₂0 H+ Н b. in Rite the complete reaction Mechaniszn For the Formation of each product. ·C. Suggest what Reaction conditions could Result in each product being the major Product of the veaction:arrow_forwarda. Write the product For each of the Following reactions H 6-836-6 레 +H₂ N A H A-C-C=C-C-CH + 2 Na +2 NH3 - H H b. Write the reaction Mechanism For. reaction eacharrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning  Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY