
Concept explainers
Interpretation: The correct name for below cyclic
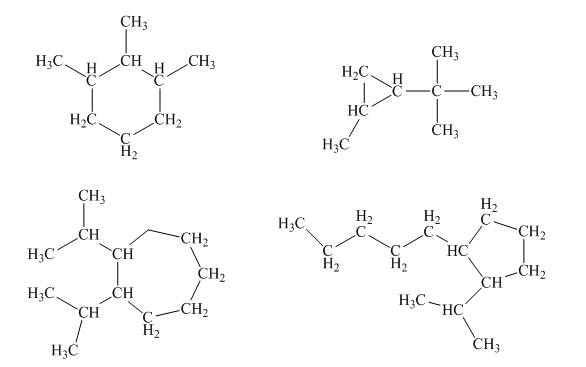
Concept introduction: Systematic way to name different organic compounds is
Rules for nomenclature of alkanes:
1 The longest continuous carbon chain is identified first and named in accordance with number of carbon atoms present in it. For example, a hydrocarbon with one carbon atom has prefix “meth”, that with two carbon atoms has prefix “eth”, that with three carbon atoms has prefix “prop” and so on. Suffix used for alkanes is “ane.”
2. Substituents attached to the parent carbon chain are to be identified. These are named by removal of single hydrogen atom from carbon chain end and named by replacement of suffix “ane” by “yl.” For example, if
3. Carbons of the parent chain are named in such a way that substituents acquire the lowest numbers.
4. If the same substituent is present more than one time in molecule, it is represented by prefix “di”, “tri” and so on. It depends on number of times substituent occurs in molecule.
5. If two or more substituents are present in the molecule, these are named in alphabetical order.
6. If in a molecule, the carbon chain of same length exists then the parent chain will be the one which contains lowest number of substituents.
7. Prefix “cyclo” is used if cyclic alkane is present in a molecule.
Trending nowThis is a popular solution!

Chapter NW1 Solutions
Custom eBook for Organic Chemistry
- Draw a Newman projection for the molecule below from the perspective indicated. Which of the groups (letters A-H) are methyl groups? CH3 H H H A H B ☑ >> H. ABCDEFG I H -H CH3 G D CH F E Numeric 4 points How many gauche interactions exist in the conformation shown in the previous problem? 1arrow_forwardHELP NOW PLEASE ! ASAP! URGENT!arrow_forwardHELP NOW PLEASE ! ASAP! URGENT!arrow_forward
- Would the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forwardPls help.arrow_forward13) When solid barium phosphate is in equilibrium with its ions, the ratio of barium ions to phosphate ions would be: a. 1:1 b. 2:3 c. 3:2 d. 2:1 14) The pH of a 0.05 M solution of HCl(aq) at 25°C is 15) The pH of a 0.20 M solution of KOH at 25°C isarrow_forward
- Pls help.arrow_forwardPls help.arrow_forward16) A 2.0 L flask containing 2.0 x 10-3 mol H2(g), 3.0 x 10-3 mol Cl2(g), and 4.0 x 10-3 mol HCl(g) at equilibrium. This system is represented by the following chemical equation: H2 (g) + Cl2 (g) → 2HCl(g) Calculate the equilibrium constant for this reaction.arrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

