
Concept explainers
(a)
Interpretation: Reason behind
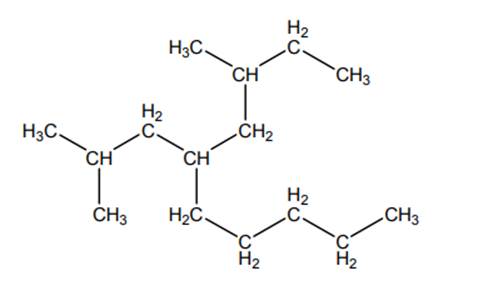
Concept introduction:The IUPAC name begins with prefix to designate the number of
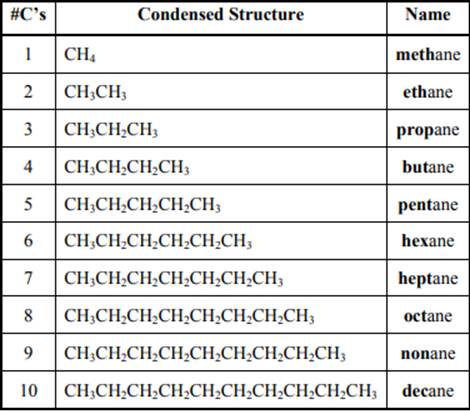
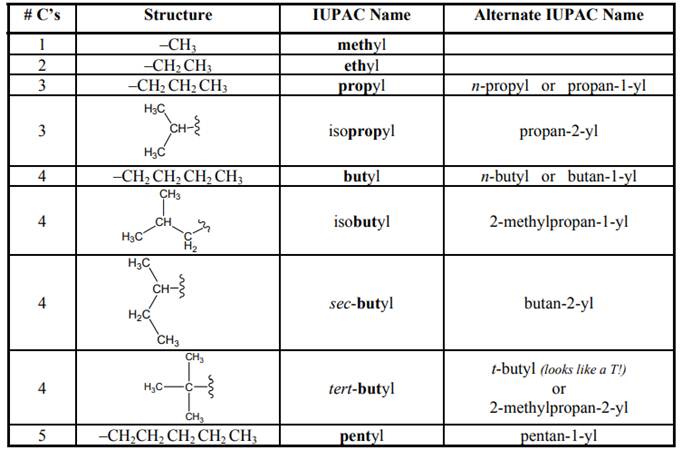
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
As per IUPAC recommendations, longest chain found in a continuous manner in a branched molecule is chosen as parent chain.
(b)
Interpretation:Reason behind
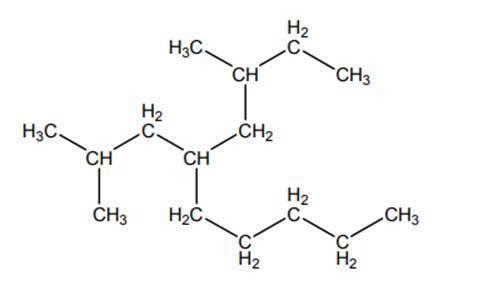
Concept introduction:The IUPAC name begins with prefix to designate the number of
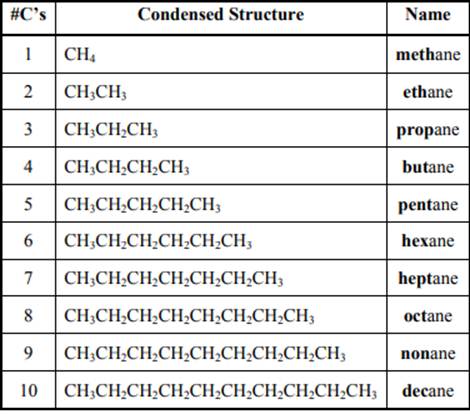
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
As per IUPAC recommendations, all the side chains are named in alphabetical order.Names of branches that occur commonly as side chains are listed below:
Trending nowThis is a popular solution!

Chapter NW1 Solutions
Custom eBook for Organic Chemistry
- What are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat are the products of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat would happen if you added the HCI to the Grignard reagent before adding benzophenone? Draw a reaction mechanism to support your answer.arrow_forward
- At 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Calculate the order of the reaction. t/s [R]/ (mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forwardWrite the correct IUPAC names of the molecules in the picturearrow_forwardHow many grams of solid NaCN have to be added to 1.5L of water to dissolve 0.18 mol of Fe(OH)3 in the form Fe(CN)63 - ? ( For simplicity, ignore the reaction of CN - ion with water) Ksp for Fe(OH)3 is 2.8E -39, and Kform for Fe(CN)63 - is 1.0E31arrow_forward
- Draw the most stable chair conformation of 1-ethyl-1-methylcyclohexane, clearly showing the axial and equatorial substituents. [4] Draw structures corresponding to the following IUPAC name for each of the following compounds; [5] i) 4-Isopropyl-2,4,5-trimethylheptane ii) trans-1-tert-butyl-4-ethylcyclohexane iii) Cyclobutylcycloheptane iv) cis-1,4-di-isopropylcyclohexane (chair conformation) v) 3-Ethyl-5-isobutylnonanearrow_forwardDraw and name molecules that meet the following descriptions; [4] a) An organic molecule containing 2 sp2 hybridised carbon and 1 sp-hybridised carbon atom. b) A cycloalkene, C7H12, with a tetrasubstituted double bond. Also answer question 2 from the imagearrow_forwardH 14. Draw the line angle form of the following molecule make sure you use the proper notation to indicate spatial positioning of atoms. F F H 15. Convert the following condensed form to line angle form: (CH3)3CCH2COCH2CON(CH2CH3)2arrow_forward
- In a reaction between two reactants A and B, the half-life is the same for both only if(A) the stoichiometry A:B is 1:1.(B) the stoichiometry A:B is 1:2 or 2:1.arrow_forwardIn a reaction between two reactants A and B, the half-life is the same for both.(1) Only if the stoichiometry A:B is 1:1.(2) If the initial quantities of A and B are in their stoichiometric ratios.arrow_forwardThere are 48 pairs of students in the following table. Each pair has quantitatively determined the mass of taurine in a 250 mL can of the popular energy drink marketed as “Munster” using High Performance Liquid Chromatography (HPLC). The class results are presented below: QUESTION: Calculate the measurement of uncertainty and provide the data in a spreadsheet table. Mass of Taurine (mg) Mass of Taurine (mg) (Table continued) 152.01 152.23 151.87 151.45 154.11 152.64 152.98 153.24 152.88 151.45 153.49 152.48 150.68 152.33 151.52 153.63 152.48 151.68 153.17 153.40 153.77 153.67 152.34 153.16 152.57 153.02 152.86 151.50 151.23 152.57 152.72 151.54 146.47 152.38 152.44 152.54 152.53 152.54 151.32 152.87 151.24 153.26 152.02 152.90 152.87 151.49 152.46 152.58arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
