
Concept explainers
(E) What does the number (+Z) at the center of each atom in Figure 1.1 represent, and whatnumber would you expect at the center of a representation of a bromine atom (Br)?
Interpretation:
The purpose of the representation of number (+Z) at the centre of each atom in given figure 1.1 should be determined along with the number which is present at the centre of a representation of bromine atom.
Concept Introduction:
In a planetary model of an atom, negative charged electrons are arranged around the positive charged electron in a series of shells which is like orbits.
The electrons present in the outermost energy level or shell is known as valence electrons. These electrons are available for bonding and outermost shell is known as valence shell.
Answer to Problem 1CTQ
+Z represents the nuclear charge of an atom which is equal to the number of protons or atomic number.
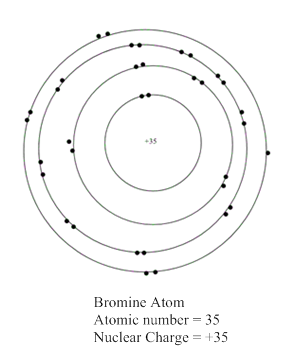
The representation of bromine atom:
Explanation of Solution
Given information:
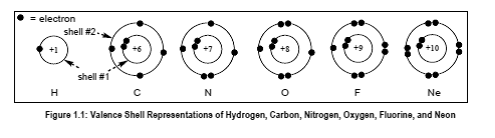
In the given figure, the number Z represents the atomic number of an atom such as atomic number of carbon is 6. +Z represents the nuclear charge which is nothing but atomic number.
The total charge present on all the protons in the nucleus is known as nuclear charge. The value of nuclear charge is equal to atomic number.
The number of protons present in nucleus of an atom is known as atomic number.
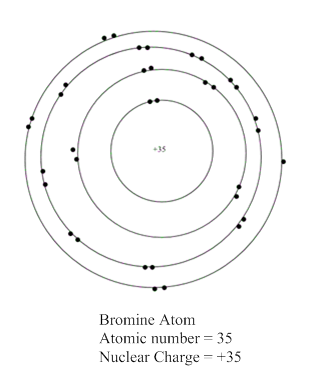
Now, atomic number of bromine atom is equal to 35 which is equal to the number of protons present in bromine atom. In the valence shell representation, +35 is present at the centre of a representation of bromine atom.
Hence, the representation of bromine atom is shown as:
Want to see more full solutions like this?
Chapter 1 Solutions
Custom eBook for Organic Chemistry
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
The Cosmic Perspective (8th Edition)
Microbiology Fundamentals: A Clinical Approach
Human Physiology: An Integrated Approach (8th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
- How can Beer’s Law be used to determine the concentration in a selected food sample. Provide an in-depth discussion and examples of this.arrow_forwardb) H3C- H3C Me CH 3 I HN Me H+arrow_forwardUsing Luther's rule, determine the reference potentials of the electrodes corresponding to the low stability systems Co³+/Co and Cr²+/Cr from the data in the table. Electrodo ΕΝ Co²+/Co Co3+/Co²+ -0,28 +1,808 Cr³+ / Cr -0,508 Cr3+ / Cr²+ -0,41arrow_forward
- The molecule PYRIDINE, 6tt electrons and is there pore aromuntre and is Assigned the Following structure contenus Since aromatk moleculey undergo electrophilic allomatic substitution, Pyridine should undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this roaction Based upon the reaction the reaction mechanism determine which of these producty would be the major Product of the hegetionarrow_forwardUsing Benzene as starting materia Show how each of the Following molecules could Ve synthesked 9. CHI d. 10450 b 0 -50311 ८ City -5034 1-0-650 e NO2arrow_forwardBA HBr of the fol 1)=MgCI 2) H₂O major NaOEt Ts Cl Py (pyridine) 1) 03 2) Me2S 1arrow_forward
- 4. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a) NHBoc ⚫OBn HO. H3C CO2CH3 -OBn H3C H3C. H3C. NHBOC CI CO2CH3arrow_forwardDraw structures of the following compounds and identify their role: mCPBA (MCPBA) DMS Py 9-BBN LAH Sia₂BH TsCI PCC t-BuOK LDA MeLi n-BuLi DMSO DMF Sodium Borohydride Lithium DiisopropylAmide 2arrow_forwardUsing Luther's rule, calculate the reference potential of the Hg2+/Hg redox electrode. DATA: Electrode potentials E° = 0,854 V y E 0,788 V Hg2+/Hg 2+ Hg2/Hgarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


