
Custom eBook for Organic Chemistry
2nd Edition
ISBN: 9798214171104
Author: Straumanis
Publisher: Cengage Custom
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 1, Problem 6CTQ
Interpretation Introduction
Interpretation:
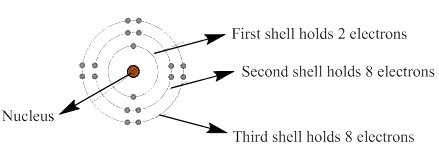
The lone pair which is shown in the first four rows of the given figure should be identified.
Concept Introduction:
In a planetary model of an atom, negatively charged electrons are arranged around the positively charged electron in a series of shells which is like orbits.
The electrons present in the outermost energy level or shell are known as valence electrons. These electrons are available for bonding and the outermost shell is known as valence shell.
The general valence shell representation is shown as:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
7
Draw the starting alkyl bromide that would produce this alkyne
under these conditions.
F
Drawing
1. NaNH2, A
2. H3O+
£
4 Temps to rise
Tomorrow
Q Search
H2
7
Comment on the general features of the predicted (extremely simplified) ¹H-
NMR spectrum of lycopene that is provided below.
00
6
57
PPM
3
2
1
0
Indicate the compound formula: dimethyl iodide (propyl) sulfonium.
Chapter 1 Solutions
Custom eBook for Organic Chemistry
Ch. 1 - (E) What does the number (+Z) at the center of...Ch. 1 - Prob. 2CTQCh. 1 - Prob. 3CTQCh. 1 - Prob. 4CTQCh. 1 - Prob. 5CTQCh. 1 - Prob. 6CTQCh. 1 - Prob. 7CTQCh. 1 - You hear a student from a nearby group say that...Ch. 1 - Use VSEPR to explain why the HBH bond angle of BH3...Ch. 1 - Both the HCH and HCO bond angles of H2CO...
Ch. 1 - Prob. 11CTQCh. 1 - Consider the following flat drawing of methane...Ch. 1 - Use VSEPR to assign a value of (close to) 109.5,...Ch. 1 - A student draws the picture of ammonia (NH3) in...Ch. 1 - Prob. 15CTQCh. 1 - How many central atoms does the molecule H2NCH3...Ch. 1 - Indicate the bond angle and shape about each...Ch. 1 - Explain how there can be two kinds of bent:...Ch. 1 - A student makes the following statement: “The...Ch. 1 - A student who missed this class needs to know how...Ch. 1 - Prob. 1ECh. 1 - Prob. 2ECh. 1 - Consider the incomplete valence shell...Ch. 1 - How many valence electrons does a neutral a. K...Ch. 1 - Consider the molecules AlCl3 (aluminum chloride)...Ch. 1 - Draw an example of a bent molecule with a bond...Ch. 1 - Label each atom marked with an arrow with the...Ch. 1 - a model of each of the following molecules: a....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning