
Custom eBook for Organic Chemistry
2nd Edition
ISBN: 9798214171104
Author: Straumanis
Publisher: Cengage Custom
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 8E
a model of each of the following molecules: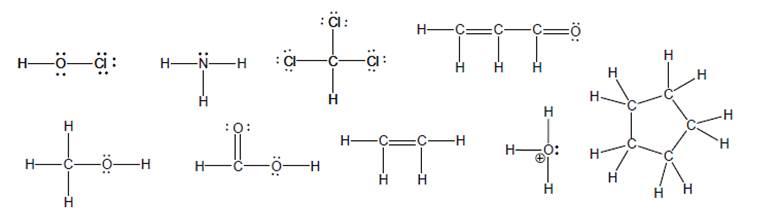
a. Based on your model, draw a bond-line representation with as many atoms as possible in the plane of the paper. Use wedge and dash bonds to represent any atoms that do not lie in the plane of the paper.
b. Indicate each unique bond angle and the shape of each unique central atom.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
From the given compound, choose the proton that best fits each given description.
a
CH2
CH 2
Cl
b
с
CH2
F
Most shielded:
(Choose one)
Least shielded:
(Choose one)
Highest chemical shift:
(Choose one)
Lowest chemical shift:
(Choose one)
×
Consider this molecule:
How many H atoms are in this molecule?
How many different signals could be found in its 1H NMR spectrum?
Note: A multiplet is considered one signal.
For each of the given mass spectrum data, identify whether the compound contains chlorine, bromine, or neither.
Compound
m/z of M* peak
m/z of M
+ 2 peak
ratio of M+ : M
+ 2 peak
Which element is present?
A
122
no M
+ 2 peak
not applicable
(Choose one)
B
78
80
3:1
(Choose one)
C
227
229
1:1
(Choose one)
Chapter 1 Solutions
Custom eBook for Organic Chemistry
Ch. 1 - (E) What does the number (+Z) at the center of...Ch. 1 - Prob. 2CTQCh. 1 - Prob. 3CTQCh. 1 - Prob. 4CTQCh. 1 - Prob. 5CTQCh. 1 - Prob. 6CTQCh. 1 - Prob. 7CTQCh. 1 - You hear a student from a nearby group say that...Ch. 1 - Use VSEPR to explain why the HBH bond angle of BH3...Ch. 1 - Both the HCH and HCO bond angles of H2CO...
Ch. 1 - Prob. 11CTQCh. 1 - Consider the following flat drawing of methane...Ch. 1 - Use VSEPR to assign a value of (close to) 109.5,...Ch. 1 - A student draws the picture of ammonia (NH3) in...Ch. 1 - Prob. 15CTQCh. 1 - How many central atoms does the molecule H2NCH3...Ch. 1 - Indicate the bond angle and shape about each...Ch. 1 - Explain how there can be two kinds of bent:...Ch. 1 - A student makes the following statement: “The...Ch. 1 - A student who missed this class needs to know how...Ch. 1 - Prob. 1ECh. 1 - Prob. 2ECh. 1 - Consider the incomplete valence shell...Ch. 1 - How many valence electrons does a neutral a. K...Ch. 1 - Consider the molecules AlCl3 (aluminum chloride)...Ch. 1 - Draw an example of a bent molecule with a bond...Ch. 1 - Label each atom marked with an arrow with the...Ch. 1 - a model of each of the following molecules: a....
Additional Science Textbook Solutions
Find more solutions based on key concepts
An electric motor has an effective resistance of 32.0 and an inductive reactance of 45.0 when working under l...
Fundamentals of Physics Extended
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used hand raiting and don't used Ai solutionarrow_forward2' P17E.6 The oxidation of NO to NO 2 2 NO(g) + O2(g) → 2NO2(g), proceeds by the following mechanism: NO + NO → N₂O₂ k₁ N2O2 NO NO K = N2O2 + O2 → NO2 + NO₂ Ко Verify that application of the steady-state approximation to the intermediate N2O2 results in the rate law d[NO₂] _ 2kk₁[NO][O₂] = dt k+k₁₂[O₂]arrow_forwardPLEASE ANSWER BOTH i) and ii) !!!!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY