
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9.8, Problem 32P
After a proton is removed from the OH group, which compound in each pair forms a cyclic ether more rapidly?

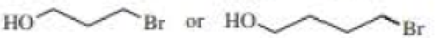
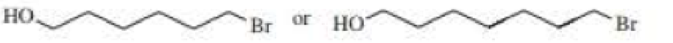
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please predict the products for each of the
following reactions.
Clearly show the regiochemistry (Markovnikov
vs anti-Markovnikov) and stereochemistry
(syn- vs anti- or both).
If a mixture of enantiomers is formed, please
draw all the enantiomers.
Hint: In this case you must choose the best
answer to demonstrate the stereochemistry of
H2 addition.
1.03
2. (CH3)2S
BIZ
CH₂OH
2. DMS
KMnO4, NaOH
ΖΗ
Pd or Pt (catalyst)
HBr
20 1
HBr
ROOR (peroxide)
HO
H-SO
HC
12 11 10
BH, THE
2. H2O2, NaOH
Brz
cold
HI
19
18
17
16
MCPBA
15
14
13
A
Br
H₂O
BH3⚫THF
Brz
EtOH
Pd or Ni (catalyst)
D₂ (deuterium)
1. Os04
2. H2O2
CH3CO3H
(peroxyacid)
1. MCPBA
2. H₂O*
H
B
+
H
H
H
"H
C
H
H
D
Explain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.
Explain the importance of having a sampling plan with respect to food analysis.
Explain the importance of having a sampling plan with respect to food analysis. Provide examples.
Chapter 9 Solutions
Organic Chemistry
Ch. 9.1 - Prob. 3PCh. 9.1 - Does increasing the energy barrier for an SN2...Ch. 9.1 - Rank the following alkyl bromides from most...Ch. 9.2 - Prob. 8PCh. 9.2 - Prob. 9PCh. 9.2 - Prob. 10PCh. 9.2 - Prob. 11PCh. 9.2 - Which substitution reaction lakes place more...Ch. 9.2 - Prob. 14PCh. 9.2 - Prob. 16P
Ch. 9.3 - Prob. 17PCh. 9.4 - Prob. 18PCh. 9.5 - Prob. 19PCh. 9.5 - Prob. 20PCh. 9.5 - Prob. 21PCh. 9.5 - Prob. 22PCh. 9.6 - Prob. 23PCh. 9.6 - Prob. 24PCh. 9.6 - Which of the following reactions take place more...Ch. 9.7 - Prob. 26PCh. 9.7 - Prob. 27PCh. 9.7 - Prob. 28PCh. 9.7 - Prob. 30PCh. 9.7 - Under which of the following reaction conditions...Ch. 9.8 - After a proton is removed from the OH group, which...Ch. 9.8 - Prob. 33PCh. 9.9 - Prob. 34PCh. 9 - Prob. 1PCh. 9 - Methoxychlor is an insecticide that was intended...Ch. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Prob. 37PCh. 9 - Prob. 38PCh. 9 - Prob. 39PCh. 9 - Prob. 40PCh. 9 - Starting with cyclohexene, how can the following...Ch. 9 - Prob. 42PCh. 9 - The pKa of acetic acid in water is 4.76. What...Ch. 9 - Prob. 44PCh. 9 - Prob. 45PCh. 9 - Prob. 46PCh. 9 - Prob. 47PCh. 9 - Prob. 48PCh. 9 - Prob. 49PCh. 9 - Prob. 50PCh. 9 - Prob. 51PCh. 9 - tert-Butyl chloride undergoes solvolysis in both...Ch. 9 - Prob. 53PCh. 9 - Prob. 54PCh. 9 - In which solventethanol or diethyl etherwould the...Ch. 9 - Prob. 56PCh. 9 - Two bromoethers are obtained from the reaction of...Ch. 9 - Prob. 58PCh. 9 - Prob. 59PCh. 9 - Prob. 60PCh. 9 - Propose a mechanism for the following reaction:Ch. 9 - Prob. 62PCh. 9 - Prob. 63PCh. 9 - Prob. 64PCh. 9 - Prob. 65PCh. 9 - When equivalent amounts of methyl bromide nod...Ch. 9 - Prob. 67PCh. 9 - The reaction of chloromethane with hydroxide ion...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forwardElectrochemistry. Briefly describe the Gibbs model and the Gibbs absorption equation.arrow_forward
- Briefly state the electrocapillary equation for ideally polarized electrodes.arrow_forwardWhat is surface excess according to the Gibbs model?arrow_forwardUsing Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forward
- The molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY