
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Question
Chapter 9, Problem 1P
Interpretation Introduction
Interpretation:
The structure of DDE is to be drawn.
Concept Introduction:
Compounds in which one or more hydrogen atoms are replaced by halogen atoms are called
These alkyl halides usually undergo substitution or addition reactions.
The electronegative atom is replaced by another atoms or group are referred as substitution reactions.
The electronegative atom is eliminated along with a hydrogen from adjacent carbon is called elimination reactions.
Expert Solution & Answer
Answer to Problem 1P
The structure of DDE is,
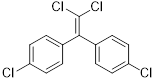
Explanation of Solution
DDE (dichlorodiphenyldichloroethylene) is a chemical compound forms as a result of loss of hydrogen chloride from DDT (dichlorodiphenyltrichloroethane).
The general reaction can be represented as:
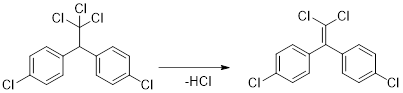
Conclusion
The structure of DDE was drawn.
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
Polymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts please.
i need help with the fol
PLEASE HELP NOW! URGENT!
Chapter 9 Solutions
Organic Chemistry
Ch. 9.1 - Prob. 3PCh. 9.1 - Does increasing the energy barrier for an SN2...Ch. 9.1 - Rank the following alkyl bromides from most...Ch. 9.2 - Prob. 8PCh. 9.2 - Prob. 9PCh. 9.2 - Prob. 10PCh. 9.2 - Prob. 11PCh. 9.2 - Which substitution reaction lakes place more...Ch. 9.2 - Prob. 14PCh. 9.2 - Prob. 16P
Ch. 9.3 - Prob. 17PCh. 9.4 - Prob. 18PCh. 9.5 - Prob. 19PCh. 9.5 - Prob. 20PCh. 9.5 - Prob. 21PCh. 9.5 - Prob. 22PCh. 9.6 - Prob. 23PCh. 9.6 - Prob. 24PCh. 9.6 - Which of the following reactions take place more...Ch. 9.7 - Prob. 26PCh. 9.7 - Prob. 27PCh. 9.7 - Prob. 28PCh. 9.7 - Prob. 30PCh. 9.7 - Under which of the following reaction conditions...Ch. 9.8 - After a proton is removed from the OH group, which...Ch. 9.8 - Prob. 33PCh. 9.9 - Prob. 34PCh. 9 - Prob. 1PCh. 9 - Methoxychlor is an insecticide that was intended...Ch. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Prob. 37PCh. 9 - Prob. 38PCh. 9 - Prob. 39PCh. 9 - Prob. 40PCh. 9 - Starting with cyclohexene, how can the following...Ch. 9 - Prob. 42PCh. 9 - The pKa of acetic acid in water is 4.76. What...Ch. 9 - Prob. 44PCh. 9 - Prob. 45PCh. 9 - Prob. 46PCh. 9 - Prob. 47PCh. 9 - Prob. 48PCh. 9 - Prob. 49PCh. 9 - Prob. 50PCh. 9 - Prob. 51PCh. 9 - tert-Butyl chloride undergoes solvolysis in both...Ch. 9 - Prob. 53PCh. 9 - Prob. 54PCh. 9 - In which solventethanol or diethyl etherwould the...Ch. 9 - Prob. 56PCh. 9 - Two bromoethers are obtained from the reaction of...Ch. 9 - Prob. 58PCh. 9 - Prob. 59PCh. 9 - Prob. 60PCh. 9 - Propose a mechanism for the following reaction:Ch. 9 - Prob. 62PCh. 9 - Prob. 63PCh. 9 - Prob. 64PCh. 9 - Prob. 65PCh. 9 - When equivalent amounts of methyl bromide nod...Ch. 9 - Prob. 67PCh. 9 - The reaction of chloromethane with hydroxide ion...
Knowledge Booster
Similar questions
- a. Determine whether each of the Followery Molecules is in the R- On the y- Configuration 1-01"/ 1-6-4 Br 4 I el Br b. Draw The Fisher projection For all the Meso compounds that can exist FOR The Following molenlearrow_forward1- Refer to the monosaccharides below to answer each of the following question(s): CH₂OH CHO CH₂OH CH₂OH 0 H- OH 0 0 HO- H H- -OH HO H HO H H OH HO- H CH₂OH H. OH HO H HO- H CH₂OH CH₂OH CH3 a. Sorbose b. Rhamnose c. Erythrulose d. Xylulose Classify each sugar by type; for example, glucose is an aldohexose. a. Xylulose is .. b. Erythrulose is . c. Sorbose is .. d. Rhamnose is .. 2- Consider the reaction below to answer the following question(s). CHO H OH CH₂OH CH₂OH HO- H HO HO + H. -OH HO OH HO. H OH OH H -OH H OH CH₂OH Q Z a. Refer to Exhibit 25-11. Place a triangle around the anomeric carbon in compound Q. Compound Z is: b. 1. the D-anomer. 2. the a-anomer. 3. the ẞ-anomer. 4. the L-anomer. c. Which anomer is the LEAST stable? d. Q and Z are cyclic examples of: a. acetals b. hemiacetals c. alditols d. hemialditolsarrow_forwardi need help identifying the four carbon oxygen bonds in the following:arrow_forward
- Imagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule HO H3N + The solution is... X O acidic OH O basic H3N-CH-C-O O neutral ○ (unknown) O acidic ○ basic CH2 CH 3-S-CH2 O neutral ○ (unknown) H3N O OH O acidic O basic Oneutral O (unknown) 0 H3N-CH-C-O CH3 CH CH3 O acidic O basic O neutral ○ (unknown) ? olo Ar BHarrow_forwardno Ai walkthroughs need other product (product in picture is wrong dont submit the same thing)arrow_forwardHow to solve this!arrow_forward
- I have a 2 mil plastic film that degrades in 22 days at 88C and 153 days at 61C what is the predicted theoretical degradation at 47C?arrow_forwardno ai walkthrougharrow_forwardI have a 2 mil plastic film that degrades after 22 days at 88C and at 61C takes 153 days. What is the failure at 47C in days.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
