
Concept explainers
(a)
Draw the
(a)
Answer to Problem 180RP
The
Explanation of Solution
Draw the
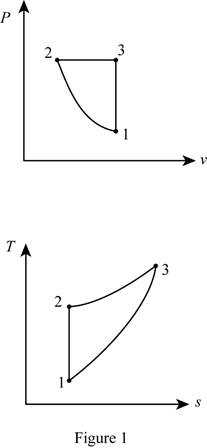
Thus, the
(b)
The expression for the back work ratio as a function of k and r.
(b)
Answer to Problem 180RP
The expression for the back work ratio as a function of k and r is
Explanation of Solution
Find the work of compression using the first law for process 1-2.
Here, heat interaction during the process 1-2 is
Write the expression of expansion work.
Here, gas constant is R, specific volume at state 2 and 3 is
Write the expression of back work ratio using the equations (I) and (II).
Here, temperature at state 1, 2, and 3 are
Conclusion:
Process 1-2: Isentropic
Calculate the ratio of
Here, pressure at state 1 and 2 is
Process 2-3: Constant pressure
Calculate the expression for
Here, volume at state 1 and 2 is
Process 3-1: Constant volume
Calculate the expression for
Substitute
Thus, the expression for the back work ratio as a function of k and r is
(c)
The expression for the cycle thermal efficiency as a function of k and r.
(c)
Answer to Problem 180RP
The expression for the cycle thermal efficiency as a function of k and r is
Explanation of Solution
Express out the heat addition and heat rejection in the process using first law to the closed system for processes 2-3 and 3-1.
Here, constant pressure specific heat is
Express the cycle thermal efficiency.
Conclusion:
Substitute
Substitute
Thus, the expression for the cycle thermal efficiency as a function of k and r is
(d)
The value of the back work ratio and thermal efficiency as r goes to unity.
(d)
Answer to Problem 180RP
The value of the back work ratio and thermal efficiency as r goes to unity is
Explanation of Solution
Recall the expression of back work ratio and apply the limits as r goes to infinity.
Thus, the value of the back work ratio as r goes to unity is
Recall the expression of cycle thermal efficiency and apply the limits as r goes to infinity.
Thus, the value of the thermal efficiency as r goes to unity is
From the results of cycle thermal efficiency and back work ratio values of 0 and 1, it shows that no expansion and net work can be done whether you add heat to the system when there is no compression
Want to see more full solutions like this?
Chapter 9 Solutions
THERMODYNAMICS (LL)-W/ACCESS >CUSTOM<
- Consider a steam power plant that operates on a simple, ideal Rankine cycle and has a net power output of 45 MW. Steam enters the turbine at 7 MPa and 500°C and is cooled in the condenser at a pressure of 10 kPa by running cooling water from a lake through the tubes of the condenser at a rate of 2000 kg/s. Show the cycle on a T-s diagram with respect to saturation lines, and determine The thermal efficiency of the cycle,The mass flow rate of the steam and the temperature rise of the cooling waterarrow_forwardTwo reversible heat engines operate in series between a source at 600°C, and a sink at 30°C. If the engines have equal efficiencies and the first rejects 400 kJ to the second, calculate: the temperature at which heat is supplied to the second engine, The heat taken from the source; and The work done by each engine. Assume each engine operates on the Carnot cyclearrow_forwardA steam turbine operates at steady state with inlet conditions of P1 = 5 bar, T1 = 320°C. Steam leaves the turbine at a pressure of 1 bar. There is no significant heat transfer between the turbine and its surroundings, and kinetic and potential energy changes between inlet and exit are negligible. If the isentropic turbine efficiency is 75%, determine the work developed per unit mass of steam flowing through the turbine, in kJ/kgarrow_forward
- Homework#5arrow_forwardMember AB has the angular velocity wAB = 2.5 rad/s and angular acceleration a AB = 9 rad/s². (Figure 1) Determine the magnitude of the velocity of point C at the instant shown. Determine the direction of the velocity of point C at the instant shown. Determine the magnitude of the acceleration of point C at the instant shown. Determine the direction of the acceleration of point C at the instant shown. A 300 mm WAB α AB B 500 mm 0=60° y 200 mmarrow_forwardYou are asked to design a unit to condense ammonia. The required condensation rate is 0.09kg/s. Saturated ammonia at 30 o C is passed over a vertical plate (10 cm high and 25 cm wide).The properties of ammonia at the saturation temperature of 30°C are hfg = 1144 ́10^3 J/kg andrv = 9.055 kg/m 3 . Use the properties of liquid ammonia at the film temperature of 20°C (Ts =10 o C):Pr = 1.463 rho_l= 610.2 kf/m^3 liquid viscosity= 1.519*10^-4 kg/ ms kinematic viscosity= 2.489*10^-7 m^2/s Cpl= 4745 J/kg C kl=0.4927 W/m Ca)Calculate the surface temperature required to achieve the desired condensation rate of 0.09 kg/s( should be 688 degrees C) b) Show that if you use a bigger vertical plate (2.5 m-wide and 0.8 m-height), the requiredsurface temperature would be now 20 o C. You may use all the properties given as an initialguess. No need to iterate to correct for Tf. c) What if you still want to use small plates because of the space constrains? One way to getaround this problem is to use small…arrow_forwardIf you have a spring mass damper system, given by m*x_double_dot + c*x_dot + kx = 0 where m, c, k (all positive scalars) are the mass, damper coefficient, and spring coefficient, respectively. x ∈ R represents the displacement of the mass. Let us then discuss the stability of the system by using Lyapunov stability theorem. Consider the system energy as a candidate Lyapunov function shown in the image. Discuss the positive definiteness of V (x, x_dot). Derive the Lyapunov rate of this system (i.e., V_dot ), and discuss the stability property of thesystem based on the information we gain from ̇V_dot .arrow_forwardIn class, two approaches—Theorems 1 and 2 below—are discussed to prove asymptotic stability of asystem when ̇V = 0. Show the asymptotic stability of the system given in Eq. (1) by applying Theorem 1. Show the asymptotic stability of the system given in Eq. (1) by applying Theorem 2.arrow_forwardHomework#5arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_iosRecommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY