
You are checking the performance of a reactor in which acetylene is produced from methane in the reaction
An undesired side reaction is the decomposition of acetylene:
Methane is fed to the reactor at 1500°C at a rate of 10.0 mol CH4/S. Heat is transferred to the reactor at a rate of 975 kW. The product temperature is 1500°C and the fractional conversion of methane is 0.600. A flowchart of the process and an enthalpy table are shown below.
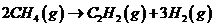
References: C(s), H2(g), at 25°C, 1 atm
| Substance |
|
|
|
|
|
|
10.0 | 41.65 |
|
|
|
|
— | — |
|
|
|
|
— | — |
|
|
| C | — | — |
|
|
(a) Using the heat capacities given below for enthalpy calculations, write and solve material balances and an energy balance to determine the product component flow rates and the yield of acetylene (mol C2H2produced/mol CH4consumed).
For example, the specific enthalpy of methane at 1500°C relative to methane at 25°C is [0.079 kJ/(mol·°C)](1500°C - 25°C) = 116.5 kJ/mol.
(b) The reactor efficiency may be defined as the ratio (actual acetylene yield/acetylene yield with no side reaction). What is the reactor efficiency for this process?
(c) The mean residence time in the reactor [
If the mean residence time increases to infinity, what would you expect to find in the product stream? Explain.
Someone proposes running the process with a much greater feed rate than the one used in Part (a), separating the products from the unconsumed reactants, and recycling the reactants. Why would you expect that process design to increase the reactor efficiency? What else would you need to know to determine whether the new design would be cost-effective?
Trending nowThis is a popular solution!

Chapter 9 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Starting Out with Python (4th Edition)
Web Development and Design Foundations with HTML5 (8th Edition)
Concepts Of Programming Languages
Problem Solving with C++ (10th Edition)
Introduction To Programming Using Visual Basic (11th Edition)
Degarmo's Materials And Processes In Manufacturing
- Nucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor” *see attachedarrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forward
- Show the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forwardDraw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forward
- Show the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forwardElimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





