
Concept explainers
Consider an
molecular orbital is promoted to the lowest empty molecular orbital. (a) Identify the molecular orbitals involved, and sketch a diagram to show the transition. (b) Compare the bond order and bond length of
Interpretation:
The molecular orbitals involved in the transition of an electron in a
Concept introduction:
Two atomic orbitals combine in order to form a bonding and an antibonding molecular orbital. The orbitals that lie on internuclear axis combine to form
The molecular orbital formed by the combination of
Molecular orbital formed by the combination of
Molecular orbitals formed by combining
Electrons are filled in the molecular orbitals in increasing order of energy.
Bond order is determined by subtracting the number of electrons in antibonding orbitals from the number of electrons in bonding orbitals and dividing by two.
Higher the bond order, smaller is the bond length.
Paramagnetic substances possess net spin and a diamagnetic substance has zero spin.
The energy of the photon emitted is given by the expression as follows:
Here,
Answer to Problem 114AP
Solution:
a) One electron of

b) Bond order of
c)
d)
Explanation of Solution
a) The molecular orbitals involved to be identified and a diagram to show the transition.
The electronic configuration of a nitrogen atom is as follows:
The molecular orbital diagram for
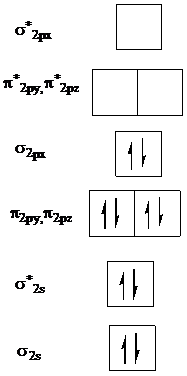
The highest occupied molecular orbital in
Thus, promote one electron of
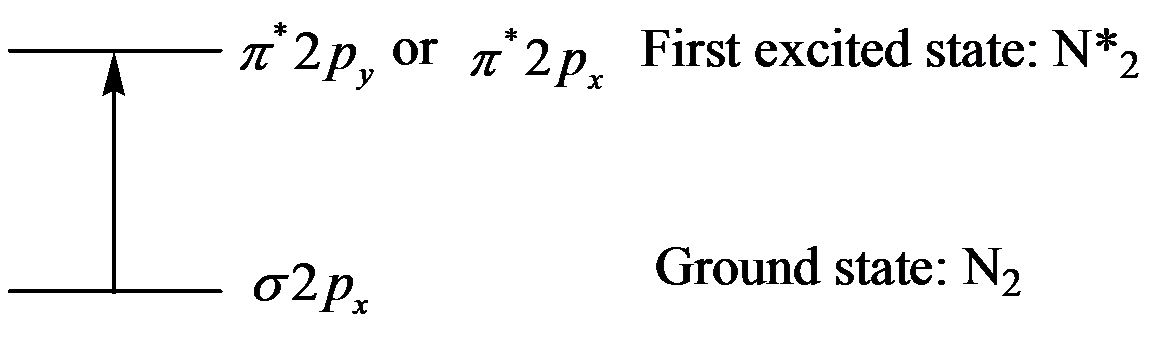
Explanation:
b) Comparison of bond order and bond length of
In
The bond order of
In
The bond order of
Hence bond order of
Thus, the bond length of
Explanation:
c)
The excited state of
Explanation:
d) The energy difference between two levels, when
The energy difference between the excited state and the ground state is equal to the energy of the photon emitted.
The energy of the photon is given by the expression as follows:
Here,
Here, the wavelength
Substitute
Hence, the energy difference between the ground state and excited state is
Want to see more full solutions like this?
Chapter 9 Solutions
Chemistry
- Calculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 Group of answer choices 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 choices: 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0arrow_forward
- Please solvearrow_forwardRank the compounds in each group below according to their reactivity toward electrophilic aromatic substitution (most reactive = 1; least reactive = 3). Place the number corresponding to the compounds' relative reactivity in the blank below the compound. a. CH₂F CH3 F b. At what position, and on what ring, is bromination of phenyl benzoate expected to occur? Explain your answer. :0: C-O phenyl benzoate 6.Consider the reaction below to answer the following questions. A B C NO₂ FeBr3 + Br₂ D a. The nucleophile in the reaction is: BODADES b. The Lewis acid catalyst in the reaction is: C. This reaction proceeds d. Draw the structure of product D. (faster or slower) than benzene.arrow_forwardPart 2. A solution of 6.00g of substance B in 100.0mL of aqueous solution is in equilibrium, at room temperature, wl a solution of B in diethyl ether (ethoxyethane) containing 25.0 g of B in 50.0 mL 9) what is the distribution coefficient of substance B b) what is the mass of B extracted by shaking 200 ml of an aqueous solution containing 10g of B with call at room temp): i) 100 mL of diethyl ether ii) 50ml of diethyl ether twice iii) 25ml of diethyl ether four timesarrow_forward
- - Rank the following groups of compounds from most acidic (1) to least acidic (4). Place the number corresponding to the compound's relative rank in the blank below the structure. a. NO₂ NO₂ CH2CH2CH2CH2OH CH3 CH3CH2CHOH CH3CH2CH2CH2OH NO₂ CH3CHCH2CH2OH b. OH OH CH₂OH CO₂H HC CN CN CNarrow_forwardGive the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry a. H MgBr 1. ether 2. H₂O* 4 COH b. 1. LIAIH, ether 2. H₂O Choose the best reagent(s) for carrying out the following conversions from the list provided below. Place the letter of the best choice in the blank to the left of the conversion. Reagents may be used more than once. a. 1. CH3MgBr, ether 2. H3O+ NaOH b. 1. PBr3 2. C. 2. 1. (CH3)3SiCl, (CH3CH2)3N CH3MgBr, ether 3. H₂O*+ 2. H3O+ e. 1. p-TosCl, pyridine f. نها g. 2. NaOH CrO3, H₂SO4, H₂O 1. NaBH4, ethanol 2. H30* h. PCC, CH2Cl2 Ovoldo-6 a. b. OH OH H OH O any organicarrow_forwardDetermine the rate law for sodium thiosulfate from the following data: [Na2S2O3] Time (s) 0.0318 230. 0.0636 57.5arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
