
Concept explainers
a)
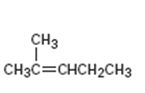
Interpretation:
The structure of the product obtained by the hydroboration-oxidation of 2-methyl-2-pentene is to be given.
Concept introduction:
The hydroboration reaction takes place with syn stereochemistry and results in a non-Markovnikov addition of water to the double bond in an
To give:
The structure of the product obtained by the hydroboration-oxidation of 2-methyl-2-pentene.
b)
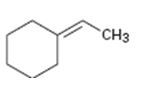
Interpretation:
The structure of the product obtained by the hydroboration-oxidation of the compound is to be given.
Concept introduction:
The hydroboration reaction takes place with syn stereochemistry and results in a non-Markovnikov addition of water to the double bond in the alkene. The resulting product has the hydroxyl group on the less highly substituted carbon.
To give:
The structure of the product obtained by the hydroboration-oxidation of the compound shown.
Trending nowThis is a popular solution!

Chapter 8 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- I performed this experiment, but I'm so confused. How do I find the first two blank columns using the data provided. What is the [I^-] mol/L and [S2O8^-2] mol/L. How do I find this? Please help!arrow_forwardExample 3 A molecule is achiral if it has a plane of symmetry in any conformation. The given conformation of 2,3-dibromobutane below does not have a plane of symmetry. Will rotation around the C2-C3 bond form a conformation with a plane of symmetry? Draw the conformation to find out. DIY: Do the same for: H3C Brill rotate H CH3 OH HO Brarrow_forward120 100 20 20 bound drug/free drug (%) 60 40 60 80 80 0 0 Scatchard Plot of Drug Binding 20 20 40 60 80 100 120 bound drug (nM)arrow_forward
- Using diethylmalonate and benzyl bromide as your only as your only source of carbon, propose a synthesis for the following compound.arrow_forwardplease helparrow_forwardWhat is the difference between (+)-(S)-methamphetamine and (-)-(R)-methamphetamine versus levo-methamphetamine and dextro-methamphetamine, D-methamphetamine, and L-methamphetamine, and N-methamphetamine? Please use scholarly sources and in-text citations.arrow_forward
- 5. A buffer consists of 0.45 M NH, and 0.25 M NH-CI (PK of NH 474) Calculate the pH of the butter. Ans: 9.52 BAS PH-9.26 +10g (10.95)) 14-4.59 PH=4.52 6. To 500 ml of the buffer on #5 a 0.20 g of sample of NaOH was added a Write the net ionic equation for the reaction which occurs b. Should the pH of the solution increase or decrease sightly? Calculate the pH of the buffer after the addition Ans: 9.54arrow_forwardExplain the inductive effect (+I and -I) in benzene derivatives.arrow_forwardThe inductive effect (+I and -I) in benzene derivatives, does it guide ortho, meta or para?arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


