
(a)
Interpretation:The effect of structure of a polymer on the melting point of the material.
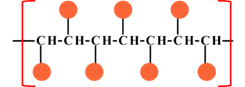 |
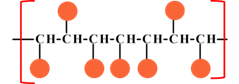 |
| Syndiotactic | Atactic |
Where |
 |
Concept introduction:The tacticity of polymer is an important property of oligomer, where the repeating unit of the polymer is a small molecule. The tacticity mainly arise on the substitution of the in line
(b)
Interpretation:The reason behind the high cost of syndiotactic polymers over atactic polymers.
Concept introduction:The tacticity of polymer is an important property of oligomer, where the repeating unit of the polymer is a small molecule. The tacticity mainly arise on the substitution of the in line polymeric unit. The arrangement of the substituted entity arise various tacticity in the polymers in the name of isotactic, syndiotactic and atactic.
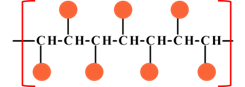 |
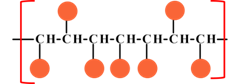 |
| Syndiotactic | Atactic |
Where |
 |
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Chemistry for Engineering Students
- Predict the major organic product for this reaction.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardPlease provide the complete mechanism for the reaction below and include all appropriate arrows, formal charges, and intermediates. Please draw out the answerarrow_forward
- 3) The following molecule, chloral is a common precursor to chloral hydrate, an acetal type molecule that was a first-generation anesthetic. Draw a mechanism that accounts for tis formation and speculate why it does not require the use of an acid catalyst, like most hemiacetal and acetal reaction: (10 pts) H H₂Oarrow_forwardYou are a Quality Manager for a very well-known food ingredient company that produces umami powder, and you are responsible for setting specification limits. The net weight (in grams) of bags of unami powder is monitored by taking samples of six bags on an hourly basis during production. The label on every bag reports a contents of 1KG umami powder. The process mean is μ = 1012 g, and when the process is properly adjusted, it varies with σ = 11 g. QUESTION: Your organisation strives to ensure that >99.97% of bags of umami powder produced conforms to specification. What performance process index value is required to achieve this process yield? Calculate PPK using the following formula: Ppk = (USL – mean)/3 σ Ppk = (mean -LSL)/ 3 σarrow_forwardYou are a Quality Manager for a very well-known food ingredient company that produces umami powder, and you are responsible for setting specification limits. The net weight (in grams) of bags of unami powder is monitored by taking samples of six bags on an hourly basis during production. The label on every bag reports a contents of 1KG umami powder. The process mean is μ = 1012 g, and when the process is properly adjusted, it varies with σ = 11 g. QUESTION: Provide a valid and full justification as to whether you would advise your manager that the process is satisfactory when it is properly adjusted, or would you seek their approval to improve the process?arrow_forward
- You are a Quality Manager for a very well-known food ingredient company that produces umami powder, and you are responsible for setting specification limits. The net weight (in grams) of bags of unami powder is monitored by taking samples of six bags on an hourly basis during production. The label on every bag reports a contents of 1KG umami powder. The process mean is μ = 1012 g, and when the process is properly adjusted, it varies with σ = 11 g. QUESTION: Using all the available information, set the upper and lower specification limits.arrow_forward43) 10.00 ml of vinegar (active ingredient is acetic acid) is titrated to the endpoint using 19.32 ml of 0.250 M sodium hydroxide. What is the molarity of acetic acid in the vinegar? YOU MUST SHOW YOUR WORK. NOTE: MA x VA = MB x VBarrow_forward424 Repon Sheet Rates of Chemical Reactions : Rate and Order of 1,0, Deception B. Effect of Temperature BATH TEMPERATURE 35'c Yol of Oh نام Time 485 Buret rend ing(n) 12 194 16. 6 18 20 10 22 24 14 115 95 14738 2158235 8:26 CMS 40148 Total volume of 0, collected Barometric pressure 770-572 ml mm Hg Vapor pressure of water at bath temperature (see Appendix L) 42.2 Slope Compared with the rate found for solution 1, there is Using the ideal gas law, calculate the moles of O; collected (show calculations) times faster 10 Based on the moles of O, evolved, calculate the molar concentration of the original 3% 1,0, solution (sho calculations)arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





