
(a)
Interpretation:
For the given organic structures IUPAC name should be identified.
Concept Introduction
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of
For
Root word represents the longest continuous carbon skeleton of the organic molecule.
When a molecule consists of cyclic structure, the root word of the molecule is prefixed with cyclo, if it is two cyclic structure combined then prefixed with bicyclo.
Two stereoisomers are there for an alkene molecule. It depends upon the location of bulky group (or high molecular weight) on the double bonded carbon atoms. If the bulky groups are in same side then it is cis-isomer. If the bulky groups are in opposite side then it is trans-isomer.
(a)
Answer to Problem 50PP
The systematic name for the molecule (a) is trans-3,4,5,5-tetramethyl-3-heptene.
Explanation of Solution
To identify: The systematic name for the given structure (a).
Draw the given molecule (a) and find the longest parent carbon chain or carbon skeleton and the substituents for naming the compound.
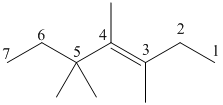
trans-3,4,5,5-tetramethyl-3-heptene
The given molecule is drawn. The parent carbon skeleton is the longest continuous carbon chain that should contain more number of carbons. In the given molecule the parent carbon skeleton is the chain which contains 7 carbons. Hence the root name of the molecule is hept. Since it is an alkene with 7 carbons then heptene will be the parent carbon chain name.
The substituents present in the molecule are methyl groups on 3, 4 and 5. Therefore on numbering the three methyl groups are present at 3, 4 and 5, 5 positions is 3, 4, 5, 5-tetramethyl.
The bulky groups attached on the opposite sides of double bonded carbon atoms, so the given alkene molecule is ‘trans’.
Hence the systematic name for the molecule (a) is trans-3, 4, 5, 5-tetramethyl-3-heptene.
(b)
Interpretation:
For the given organic structures IUPAC name should be identified.
Concept Introduction
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an alkene, alkyne, alcohol, carboxylic acid, alcohol etc...
For alkenes, suffix will be ‘ene’.
Root word represents the longest continuous carbon skeleton of the organic molecule.
When a molecule consists of cyclic structure, the root word of the molecule is prefixed with cyclo, if it is two cyclic structure combined then prefixed with bicyclo.
Two stereoisomers are there for an alkene molecule. It depends upon the location of bulky group (or high molecular weight) on the double bonded carbon atoms. If the bulky groups are in same side then it is cis-isomer. If the bulky groups are in opposite side then it is trans-isomer.
(b)
Answer to Problem 50PP
The systemic name for the molecule (b) is 1-ethylcyclohexene.
Explanation of Solution
To identify: The systematic name for the given structure (b).
Draw the given molecule (b) and find the longest parent carbon chain or carbon skeleton and the substituents.
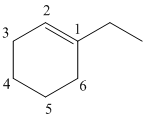
1-ethylcyclohexene
The given molecule is drawn. The parent carbon skeleton is the longest continuous carbon chain that should contain more number of carbons. In the given molecule the parent carbon skeleton is cyclic in nature which consists of 6 carbons so the root word for the molecule is cyclohexane. The suffix ‘ene’ is used since it is an alkene.
The substituent present in the molecule are 1 ethyl group. Therefore it named as 1-ethyl in suffix.
Hence the systematic name for the molecule (b) is 1-ethylcyclohexene.
(c)
Interpretation:
For the given organic structures IUPAC name should be identified.
Concept Introduction
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an alkene, alkyne, alcohol, carboxylic acid, alcohol etc...
For alkenes, suffix will be ‘ene’.
Root word represents the longest continuous carbon skeleton of the organic molecule.
When a molecule consists of cyclic structure, the root word of the molecule is prefixed with cyclo, if it is two cyclic structure combined then prefixed with bicyclo.
Two stereoisomers are there for an alkene molecule. It depends upon the location of bulky group (or high molecular weight) on the double bonded carbon atoms. If the bulky groups are in same side then it is cis-isomer. If the bulky groups are in opposite side then it is trans-isomer.
(c)
Answer to Problem 50PP
The systematic name for molecule (c) is 2–methyl bicyclo [2 2 2] oct-2-ene.
Explanation of Solution
To identify: The systematic name for the given structure (c).
Draw the given molecule (d) and find the longest parent carbon chain or carbon skeleton and the substituents.
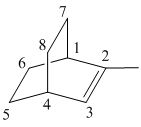
2–methylbicyclo [2 2 2] oct-2-ene
The given molecule is drawn. The parent carbon skeleton is the longest continuous carbon chain that should contain more number of carbons. In the given molecule the parent carbon skeleton is bicyclic in nature which consists of 8 carbons so the root word for the molecule is bicyclooctane. The suffix ‘ene’ is used since it is an alkene.
The given molecule is bicyclo fused alkene, number of carbons at the bridge and bridge head is counted and it is found that there are 2 carbons each at bridge head and 2 carbons at bridge.
The substituents present in the molecule are 1 methyl group. Therefore it named as 1-methyl in suffix.
Hence the systematic name for the molecule (c) is 2–methylbicyclo [2 2 2] oct-2-ene.
The systematic name for the given molecule is given by using the IUPAC rules.
Want to see more full solutions like this?
Chapter 8 Solutions
ORG.CHEM EBOOK W/BBWILEY PLUS>CUSTOM<
- 2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forwardIdentify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forward
- Instructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forwardе. Д CH3 D*, D20arrow_forwardC. NaOMe, Br Brarrow_forward
- Please predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forwardin the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forwardIs the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





