
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7, Problem 57QAP
Give the hybridization of each atom (except H) in the solvent dimethylsulfoxide. (Unshared electron pairs are not shown.)
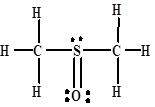
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
For the photochemical halogenation reaction below, draw both propagation steps and include the mechanism arrows for each step.
H
CH
ot
CH3
CI-CI
MM
hv
of
CH
H-CI
CH3
2nd attempt
See Periodic Table See Hint
Draw only radical electrons; do not add lone pair electrons. Note that arrows cannot meet in "space," and must end at either bonds or at
atoms.
1
i Add the missing curved arrow notation to this propagation step.
20
H
ن
S
F
P
H
CI
Br
品
The radical below can be stabilized by resonance.
4th attempt
Draw the resulting resonance structure.
DOCE
Use curved arrows to generate a second resonance form for the allylic radical formed from 2-methyl-2-pentene.
1
Draw the curved arrows that would generate a second resonance form for this radical.
D 2
H
S
F
A
Бг
I
Chapter 7 Solutions
Chemistry: Principles and Reactions
Ch. 7 - Prob. 1QAPCh. 7 - Prob. 2QAPCh. 7 - Follow the directions of Question 1 for (a) IO2-...Ch. 7 - Follow the directions of Question 1 for (a) CIF4-...Ch. 7 - Follow the directions of Question 1 for (a) OCl2...Ch. 7 - Follow the directions of Question 1 for (a) C22-...Ch. 7 - Oxalic acid, H2C2O4, is a poisonous compound found...Ch. 7 - Radio astronomers have detected the isoformyl ion,...Ch. 7 - Draw Lewis structures for the following species....Ch. 7 - Follow the directions of Question 9 for the...
Ch. 7 - Dinitrogen pentoxide, N2O5, when bubbled into...Ch. 7 - Formic acid is the irritating substance that gets...Ch. 7 - Two different molecules have the formula C2H2Cl2....Ch. 7 - Two different molecules have the formula C2H6O....Ch. 7 - Prob. 15QAPCh. 7 - Prob. 16QAPCh. 7 - Write a Lewis structure for (a) XeF3+ (b) PCl4+...Ch. 7 - Write a Lewis structure for (a) BCl4 (b) ClO- (c)...Ch. 7 - Write reasonable Lewis structures for the...Ch. 7 - Write reasonable Lewis structures for the...Ch. 7 - Prob. 21QAPCh. 7 - Draw resonance structures for (a) SeO3 (b) CS32-...Ch. 7 - Prob. 23QAPCh. 7 - Prob. 24QAPCh. 7 - The skeleton structure for disulfur dinitride,...Ch. 7 - Borazine, B3N3H6, has the skeleton Draw the...Ch. 7 - What is the formal charge on the indicated atom in...Ch. 7 - Prob. 28QAPCh. 7 - Below are two different Lewis structures for...Ch. 7 - Below are two different Lewis structures for the...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Give all the ideal bond angles (109.5, 120, or...Ch. 7 - Prob. 38QAPCh. 7 - Prob. 39QAPCh. 7 - An objectionable component of smog is acetyl...Ch. 7 - The uracil molecule is one of the bases in DNA....Ch. 7 - Niacin is one of the B vitamins (B3). Estimate the...Ch. 7 - Which of the species with octets in Question 31...Ch. 7 - Which of the species with octets in Question 32...Ch. 7 - Which of the species with octets in Question 33...Ch. 7 - Prob. 46QAPCh. 7 - There are three compounds with the formula...Ch. 7 - There are two different molecules with the formula...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - In each of the following polyatomic ions, the...Ch. 7 - Follow the directions of Question 55 for the...Ch. 7 - Give the hybridization of each atom (except H) in...Ch. 7 - Acrylonitrile, C3H3N is the building mer Orlon....Ch. 7 - What is the hybridization of nitrogen inCh. 7 - What is the hybridization of carbon inCh. 7 - Give the hybridization of the central atom...Ch. 7 - Give the hybridization of the central atom...Ch. 7 - Give the number of sigma and pi bonds in the...Ch. 7 - Give the number of sigma and pi bonds in the...Ch. 7 - Give the number of sigma and pi bonds in each...Ch. 7 - Give the number of sigma and pi bonds in each...Ch. 7 - In which of the following molecules does the...Ch. 7 - Consider the pyrosulfate ion, S2O72-. It has no...Ch. 7 - Consider acetyl salicylic acid, better known as...Ch. 7 - Complete the table on next page.Ch. 7 - Given the following electro negativities...Ch. 7 - Based on the concept of formal charge, what is the...Ch. 7 - Describe the geometry of the species in which...Ch. 7 - Consider the following molecules: SiH4, PH3, H2S....Ch. 7 - Prob. 75QAPCh. 7 - In each of the following molecules, a central atom...Ch. 7 - Prob. 77QAPCh. 7 - A compound of chlorine and fluorine, CIFx, reacts...Ch. 7 - Draw the Lewis structure and describe the geometry...Ch. 7 - Consider the polyatomic ion IO65-. How many pairs...Ch. 7 - It is possible to write a simple Lewis structure...Ch. 7 - Phosphoryl chloride, POCl3, has the skeleton...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the resulting product(s) from the coupling of the given radicals. Inlcude all applicable electrons and non-zero formal charges. H.C öö- CH3 2nd attempt +1 : 招 H₂C CH CH₂ See Periodic Table See H H C S F P Br CH₂ Iarrow_forwardPlease, help me out with the calculation, step by step on how to find what's blank with the given information.arrow_forwardPredict the following products. Then show the mechanism. H₂N NH2arrow_forward
- BF3, Boron Trifluoride, known to contain three covalent boron-fluorine bonds. suggest and illustrate all of the processes as well as their energetical consequences for the formation of BF3 from its elements.arrow_forwardDraw the mechanism of the reaction.arrow_forward9. Draw all of the possible Monochlorination Products that would Result From the Free Radical Chlormation OF 23,4-TRIMethyl Pentane b. Calculate the To Yield For the major • Product given the Following Relative Restritus For 1° 2° and 30 Hydrogens toward Free Radical Chloration 5.0: 38 : 1 30 2° 1° C. what would be the major product in the Free Radical brominator Of the Same Molecule. Explain your Reasoning.arrow_forward
- What is the complete reaction mechanism for the chlorination of Ethane, C2H6?arrow_forwardA 13C NMR spectrum is shown for a molecule with the molecular formula of C6H100. Draw the structure that best fits this data. 220 200 180 160 140 120100 80 60 40 20 Drawingarrow_forwardPlease help me figure out the blan areas with step by step calculations.arrow_forward
- needing help draw all of the possible monochlorination products that would result from the free radical chlorination of 2,3,4-trimethylpentanearrow_forwardHAND DRAWarrow_forwardBased on the 1H NMR, 13C NMR, DEPT 135 NMR and DEPT 90 NMR, provide a reasoning step and arrive at the final structure of an unknown organic compound containing 7 carbons. Dept 135 shows peak to be positive at 128.62 and 13.63 Dept 135 shows peak to be negative at 130.28, 64.32, 30.62 and 19.10. Provide assignment for the provided structurearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Linear Combination of Atomic Orbitals LCAO; Author: Edmerls;https://www.youtube.com/watch?v=nq1zwrAIr4c;License: Standard YouTube License, CC-BY
Quantum Molecular Orbital Theory (PChem Lecture: LCAO and gerade ungerade orbitals); Author: Prof Melko;https://www.youtube.com/watch?v=l59CGEstSGU;License: Standard YouTube License, CC-BY