
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 42QAP
Niacin is one of the B vitamins (B3). Estimate the approximate values of the indicated bond angles. Its skeleton (not its Lewis structure) is given below.
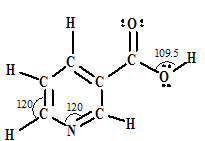
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Rank the following compounds most to least acidic:
a)
О
OH
요애
OH
.OH
flow flow
О
F
F
F
F
OH
F
b)
Ha
EN-Ha
CI
Ha
F
F CI
Ha
a)
b)
Provide arrows to show the mechanisms and then predict the products of the following acid
base reaction. Use pKas to determine which way the reaction will favor (Hint: the lower pka
acid will want to dissociate)
Дон
OH
Ha
OH
NH2
c)
H
H-O-H
MATERIALS. Differentiate between interstitial position and reticular position.
Chapter 7 Solutions
Chemistry: Principles and Reactions
Ch. 7 - Prob. 1QAPCh. 7 - Prob. 2QAPCh. 7 - Follow the directions of Question 1 for (a) IO2-...Ch. 7 - Follow the directions of Question 1 for (a) CIF4-...Ch. 7 - Follow the directions of Question 1 for (a) OCl2...Ch. 7 - Follow the directions of Question 1 for (a) C22-...Ch. 7 - Oxalic acid, H2C2O4, is a poisonous compound found...Ch. 7 - Radio astronomers have detected the isoformyl ion,...Ch. 7 - Draw Lewis structures for the following species....Ch. 7 - Follow the directions of Question 9 for the...
Ch. 7 - Dinitrogen pentoxide, N2O5, when bubbled into...Ch. 7 - Formic acid is the irritating substance that gets...Ch. 7 - Two different molecules have the formula C2H2Cl2....Ch. 7 - Two different molecules have the formula C2H6O....Ch. 7 - Prob. 15QAPCh. 7 - Prob. 16QAPCh. 7 - Write a Lewis structure for (a) XeF3+ (b) PCl4+...Ch. 7 - Write a Lewis structure for (a) BCl4 (b) ClO- (c)...Ch. 7 - Write reasonable Lewis structures for the...Ch. 7 - Write reasonable Lewis structures for the...Ch. 7 - Prob. 21QAPCh. 7 - Draw resonance structures for (a) SeO3 (b) CS32-...Ch. 7 - Prob. 23QAPCh. 7 - Prob. 24QAPCh. 7 - The skeleton structure for disulfur dinitride,...Ch. 7 - Borazine, B3N3H6, has the skeleton Draw the...Ch. 7 - What is the formal charge on the indicated atom in...Ch. 7 - Prob. 28QAPCh. 7 - Below are two different Lewis structures for...Ch. 7 - Below are two different Lewis structures for the...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Give all the ideal bond angles (109.5, 120, or...Ch. 7 - Prob. 38QAPCh. 7 - Prob. 39QAPCh. 7 - An objectionable component of smog is acetyl...Ch. 7 - The uracil molecule is one of the bases in DNA....Ch. 7 - Niacin is one of the B vitamins (B3). Estimate the...Ch. 7 - Which of the species with octets in Question 31...Ch. 7 - Which of the species with octets in Question 32...Ch. 7 - Which of the species with octets in Question 33...Ch. 7 - Prob. 46QAPCh. 7 - There are three compounds with the formula...Ch. 7 - There are two different molecules with the formula...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - In each of the following polyatomic ions, the...Ch. 7 - Follow the directions of Question 55 for the...Ch. 7 - Give the hybridization of each atom (except H) in...Ch. 7 - Acrylonitrile, C3H3N is the building mer Orlon....Ch. 7 - What is the hybridization of nitrogen inCh. 7 - What is the hybridization of carbon inCh. 7 - Give the hybridization of the central atom...Ch. 7 - Give the hybridization of the central atom...Ch. 7 - Give the number of sigma and pi bonds in the...Ch. 7 - Give the number of sigma and pi bonds in the...Ch. 7 - Give the number of sigma and pi bonds in each...Ch. 7 - Give the number of sigma and pi bonds in each...Ch. 7 - In which of the following molecules does the...Ch. 7 - Consider the pyrosulfate ion, S2O72-. It has no...Ch. 7 - Consider acetyl salicylic acid, better known as...Ch. 7 - Complete the table on next page.Ch. 7 - Given the following electro negativities...Ch. 7 - Based on the concept of formal charge, what is the...Ch. 7 - Describe the geometry of the species in which...Ch. 7 - Consider the following molecules: SiH4, PH3, H2S....Ch. 7 - Prob. 75QAPCh. 7 - In each of the following molecules, a central atom...Ch. 7 - Prob. 77QAPCh. 7 - A compound of chlorine and fluorine, CIFx, reacts...Ch. 7 - Draw the Lewis structure and describe the geometry...Ch. 7 - Consider the polyatomic ion IO65-. How many pairs...Ch. 7 - It is possible to write a simple Lewis structure...Ch. 7 - Phosphoryl chloride, POCl3, has the skeleton...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each of the following, indicate whether the arrow pushes are valid. Do we break any rules via the arrows? If not, indicate what is incorrect. Hint: Draw the product of the arrow and see if you still have a valid structure. a. b. N OH C. H N + H d. e. f. مه N COHarrow_forwardDecide which is the most acidic proton (H) in the following compounds. Which one can be removed most easily? a) Ha Нь b) Ha Нь c) CI CI Cl Ha Ньarrow_forwardProvide all of the possible resonanse structures for the following compounds. Indicate which is the major contributor when applicable. Show your arrow pushing. a) H+ O: b) c) : N :O : : 0 d) e) Оarrow_forward
- Draw e arrows between the following resonance structures: a) b) : 0: :0: c) :0: N t : 0: بار Narrow_forwardDraw the major substitution products you would expect for the reaction shown below. If substitution would not occur at a significant rate under these conditions, check the box underneath the drawing area instead. Be sure you use wedge and dash bonds where necessary, for example to distinguish between major products. Note for advanced students: you can assume that the reaction mixture is heated mildly, somewhat above room temperature, but strong heat or reflux is not used. Cl Substitution will not occur at a significant rate. Explanation Check :☐ O-CH + Х Click and drag to start drawing a structure.arrow_forwardDraw the major substitution products you would expect for the reaction shown below. If substitution would not occur at a significant rate under these conditions, check the box underneath the drawing area instead. Be sure you use wedge and dash bonds where necessary, for example to distinguish between major products. Note for advanced students: you can assume that the reaction mixture is heated mildly, somewhat above room temperature, but strong heat or reflux is not used. Cl C O Substitution will not occur at a significant rate. Explanation Check + O-CH3 Х Click and drag to start drawing a structure.arrow_forward
- ✓ aw the major substitution products you would expect for the reaction shown below. If substitution would not occur at a significant rate under these conditions, check the box underneath the drawing area instead. Be sure you use wedge and dash bonds where necessary, for example to distinguish between major products. Note for advanced students: you can assume that the reaction mixture is heated mildly, somewhat above room temperature, but strong heat or reflux is not used. C Cl HO–CH O Substitution will not occur at a significant rate. Explanation Check -3 ☐ : + D Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Cearrow_forwardPlease correct answer and don't used hand raitingarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Determine whether the following reaction is an example of a nucleophilic substitution reaction: Br OH HO 2 -- Molecule A Molecule B + Br 义 ollo 18 Is this a nucleophilic substitution reaction? If this is a nucleophilic substitution reaction, answer the remaining questions in this table. Which of the reactants is referred to as the nucleophile in this reaction? Which of the reactants is referred to as the organic substrate in this reaction? Use a ŏ + symbol to label the electrophilic carbon that is attacked during the substitution. Highlight the leaving group on the appropriate reactant. ◇ Yes O No O Molecule A Molecule B Molecule A Molecule B टेarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY