
Concept explainers
(a)
Interpretation:
The E1 product for the following reaction should be written.
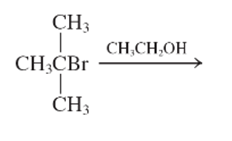
Concept Introduction:
A type of organic reaction in which two substituents get eliminated from a given molecule by one step mechanism or two step mechanism is known as elimination reaction. The reaction which occurs in one step is known as E2 reaction whereas the reaction which occurs in two steps is known as E1 reaction.
In E1 reaction, elimination of HX substituent takes place and produces a double bond. It is a unimolecular reaction.
(b)
Interpretation:
The E1 product for the following reaction should be written.
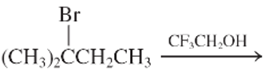
Concept Introduction:
A type of organic reaction in which two substituents get eliminated from a given molecule by one step mechanism or two step mechanism is known as elimination reaction. The reaction which occurs in one step is known as E2 reaction whereas the reaction which occurs in two steps is known as E1 reaction.
In E1 reaction, elimination of HX substituent takes place and produces a double bond. It is a unimolecular reaction.
(c)
Interpretation:
The E1 product for the following reaction should be written.
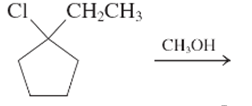
Concept Introduction:
A type of organic reaction in which two substituents get eliminated from a given molecule by one step mechanism or two step mechanism is known as elimination reaction. The reaction which occurs in one step is known as E2 reaction whereas the reaction which occurs in two steps is known as E1 reaction.
In E1 reaction, elimination of HX substituent takes place and produces a double bond. It is a unimolecular reaction.
(d)
Interpretation:
The E1 product for the following reaction should be written.
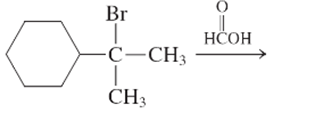
Concept Introduction:
A type of organic reaction in which two substituents get eliminated from a given molecule by one step mechanism or two step mechanism is known as elimination reaction. The reaction which occurs in one step is known as E2 reaction whereas the reaction which occurs in two steps is known as E1 reaction.
In E1 reaction, elimination of HX substituent takes place and produces a double bond. It is a unimolecular reaction.
(e)
Interpretation:
The E1 product for the following reaction should be written.
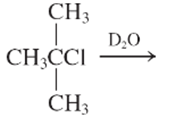
Concept Introduction:
A type of organic reaction in which two substituents get eliminated from a given molecule by one step mechanism or two step mechanism is known as elimination reaction. The reaction which occurs in one step is known as E2 reaction whereas the reaction which occurs in two steps is known as E1 reaction.
In E1 reaction, elimination of HX substituent takes place and produces a double bond. It is a unimolecular reaction.
(f)
Interpretation:
The E1 product for the following reaction should be written.
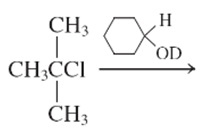
Concept Introduction:
A type of organic reaction in which two substituents get eliminated from a given molecule by one step mechanism or two step mechanism is known as elimination reaction. The reaction which occurs in one step is known as E2 reaction whereas the reaction which occurs in two steps is known as E1 reaction.
In E1 reaction, elimination of HX substituent takes place and produces a double bond. It is a unimolecular reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
EBK ORGANIC CHEMISTRY
- (10 pts) The density of metallic copper is 8.92 g cm³. The structure of this metal is cubic close-packed. What is the atomic radius of copper in copper metal?arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward
- Q3: Rank the following compounds in increasing reactivity of E1 and E2 eliminations, respectively. Br ca. go do A CI CI B C CI Darrow_forwardQ5: Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2). H₂O דיי "Br KN3 CH3CH2OH NaNH2 NH3 Page 3 of 6 Chem 0310 Organic Chemistry 1 HW Problem Sets CI Br excess NaOCH 3 CH3OH Br KOC(CH3)3 DuckDuckGarrow_forwardQ4: Circle the substrate that gives a single alkene product in a E2 elimination. CI CI Br Brarrow_forward
- Please calculate the chemical shift of each protonsarrow_forwardQ1: Answer the questions for the reaction below: ..!! Br OH a) Predict the product(s) of the reaction. b) Is the substrate optically active? Are the product(s) optically active as a mix? c) Draw the curved arrow mechanism for the reaction. d) What happens to the SN1 reaction rate in each of these instances: 1. Change the substrate to Br 'CI 2. Change the substrate to 3. Change the solvent from 100% CH3CH2OH to 10% CH3CH2OH + 90% DMF 4. Increase the substrate concentration by 3-fold.arrow_forwardQ6: Provide the reagents and conditions for the following reactions to make the product with a good yield. Br Br CI она CIarrow_forward
- Q2: We would not expect the following primary alkyl halide to go through an SN1 reaction. However, it can go through an SN1 mechanism. Explain why. Hint: Think about what happens when the leaving group leaves. CI NaO EtOH H བྱིས་ Harrow_forwardI performed this experiment, but I'm so confused. How do I find the first two blank columns using the data provided. What is the [I^-] mol/L and [S2O8^-2] mol/L. How do I find this? Please help!arrow_forwardExample 3 A molecule is achiral if it has a plane of symmetry in any conformation. The given conformation of 2,3-dibromobutane below does not have a plane of symmetry. Will rotation around the C2-C3 bond form a conformation with a plane of symmetry? Draw the conformation to find out. DIY: Do the same for: H3C Brill rotate H CH3 OH HO Brarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

