
Concept explainers
Interpretation:A reasonable rate expression corresponding to chlorination of methane reaction should be proposed.
Concept introduction:The mechanism for monochlorination comprises of three stages illustrated as follows:
Step 1- Initiation via homolytic cleavage of
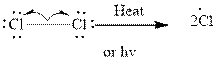
Step 2: Propagation: In the first of the propagation steps
radical generated from step 1 abstracts hydrogen radical from methane as follows:
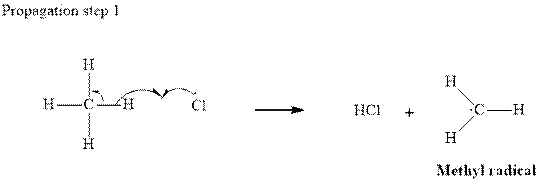
In subsequent propagation step chloromethyl radical abstracts
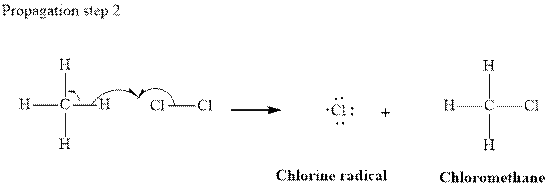
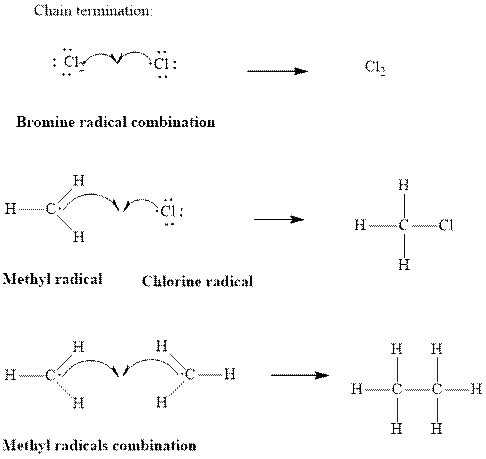
Step3: Termination: Radicals generated in propagation steps get quenched upon the combination with one another. Thus termination steps are essentially the radical− radical combination illustrated as follows:
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
EBK ORGANIC CHEMISTRY
- 2) Draw the correct chemical structure (using line-angle drawings / "line structures") from their given IUPAC name: a. (E)-1-chloro-3,4,5-trimethylhex-2-ene b. (Z)-4,5,7-trimethyloct-4-en-2-ol C. (2E,6Z)-4-methylocta-2,6-dienearrow_forwardපිපිම Draw curved arrows to represent the flow of electrons in the reaction on the left Label the reactants on the left as either "Acid" or "Base" (iii) Decide which direction the equilibrium arrows will point in each reaction, based on the given pk, values (a) + H-O H 3-H + (c) H" H + H****H 000 44-00 NH₂ (e) i Дон OH Ө NHarrow_forward3) Label the configuration in each of the following alkenes as E, Z, or N/A (for non-stereogenic centers). 00 E 000 N/A E Br N/A N/A (g) E N/A OH E (b) Oz N/A Br (d) 00 E Z N/A E (f) Oz N/A E (h) Z N/Aarrow_forward
- 6) Fill in the missing Acid, pKa value, or conjugate base in the table below: Acid HCI Approximate pK, -7 Conjugate Base H-C: Hydronium (H₂O') -1.75 H-O-H Carboxylic Acids (RCOOH) Ammonium (NH4) 9.24 Water (H₂O) H-O-H Alcohols (ROH) RO-H Alkynes R--H Amines 25 25 38 HOarrow_forward5) Rank the following sets of compounds in order of decreasing acidity (most acidic to least acidic), and choose the justification(s) for each ranking. (a) OH V SH я вон CH most acidic (lowst pKa) least acidic (highest pKa) Effect(s) Effect(s) Effect(s) inductive effect O inductive effect O inductive effect electronegativity electronegativity O electronegativity resonance polarizability resonance polarizability O resonance O polarizability hybridization Ohybridization O hybridization оarrow_forwardHow negatively charged organic bases are formed.arrow_forward
- Nonearrow_forward1) For the following molecules: (i) Label the indicated alkenes as either cis (Z), trans (E), or N/A (for non-stereogenic centers) by bubbling in the appropriate label on the molecule. (ii) Complete the IUPAC name located below the structure (HINT: Put the letter of the configuration in parentheses at the beginning of the name!) E z N/A ()-3,4,6-trimethylhept-2-ene E Oz O N/A ()-3-ethyl-1-fluoro-4-methylhex-3-ene E -+- N/A Me )-2,3-dimethylpent-2-ene (d) (b) E O N/A Br ()-5-bromo-1-chloro-3-ethyloct-4-ene ОЕ Z N/A Et (___)-3-ethyl-4-methylhex-3-ene E (f) Oz N/A z N/A HO (4.7)-4-(2-hydroxyethyl)-7-methylnona-4,7-dien-2-onearrow_forwardO 9:21AM Tue Mar 4 ## 64% Problem 51 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H :0: CI. AI :CI: :CI: Cl AI Select to Add Arrows Select to Add Arrows O: Cl :CI: :0: H CI: CI CO Select to Add Arrows Select to Add Arrows :O: CI :0: Cl. 10: AIarrow_forward
- (i) Draw in the missing lone pair(s) of electrons of the reactants on the left (ii) Draw (curved) arrows to show the flow of electrons in the acid/base reaction on the left (iii) Draw the products of the acid/base on the right (iv) Select the correct label for each product as either "conjugate acid" or "conjugate base" (a) JOH OH NH₂ acid base (b) De "H conjugate acid conjugate acid conjugate base conjugate base acid base conjugate acid conjugate base conjugate acid conjugate base acid basearrow_forwardCould someone answer this NMR and explain please Comment on the general features of the 1H-NMR spectrum of isoamyl ester provided below.arrow_forwardMacmillan Learning Draw the acyl chloride that would give the ketone shown using the Friedel-Crafts acylation reaction. Select Draw Templates More с H о Cl 2Q Erase AICI₂arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





