
(a)
Interpretation: The value of
Concept introduction:Thermodynamics is a study of energy transfers that can be done by either heat or work. The energy transferred through work involves force. When work is positive then system gains energy while when work is negative then system loses energy. Heat is not a state function and therefore change in enthalpy of reaction
(b)
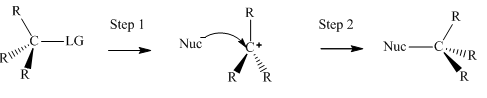
Interpretation: The mechanism using curved arrows should be indicated for below reactions.
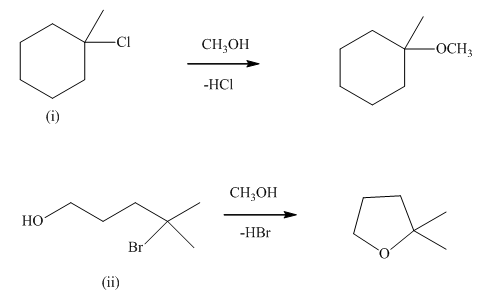
Concept introduction:Unimolecuar substitution or
A general
(c)
Interpretation: The effect observed on the rate of methanolysis when the concentration of tertiary
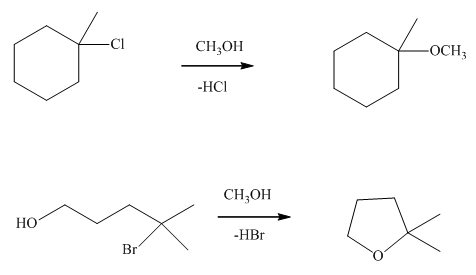
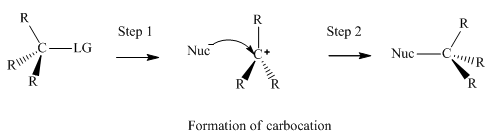
Concept introduction:Unimolecuar substitution or
A general
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
EBK ORGANIC CHEMISTRY
- The following product was made from diethyl ketone and what other reagent(s)? £ HO 10 2-pentyne 1-butyne and NaNH2 ☐ 1-propanol ☐ pyridine butanal ☐ pentanoatearrow_forwardWhich pair of reagents will form the given product? OH X + Y a. CH3 b. CH2CH3 ༧་་ C. CH3- CH2CH3 d.o6.(རི॰ e. CH3 OCH2CH3 -MgBr f. CH3-MgBr g. CH3CH2-MgBr -C-CH3 CH2CH3arrow_forwardQuestion 3 What best describes the product of the following reaction? 1. CH3CH2MgBr (2 eq) 2. H a new stereocenter will not be formed a new stereocenter will be formed an alkyl halide will result an alkane will result an aromatic compound will result 1 ptsarrow_forward
- Rank the following from most to least reactive toward nucleophilic attack. 1. [Select] [Select] 2. Acyl halide Aldehyde 3. Carboxylate ion 4. Carboxylic acid Ketone 5. [Select]arrow_forwardQuestion 10 1 pts Which of the following is the most accurate nomenclature? 1-hydroxy-1-methyldecane-4,7-dione 2-hydroxy-2-methyldecane-5,8-dione 4,6-dioxo-2-methyldecane-2-ol 9-hydroxy-9-methyldecane-3,6-dione 8-hydroxy-8-methylnonane-3,6-dione OHarrow_forwardCould you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.arrow_forward
- What are the most proper reagents to achieve these products? سد 1. 2. OH ○ 1. BrMgC6H6; 2. H+ ○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+ O 1. CH3CH2CHO; 2. H+ O 1. BrMgCH2CH3; 2. H+arrow_forwardProvide the IUPAC (systematic) name only for the following compound. Dashes, commas, and spaces must be correct. Harrow_forwardPlease use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet. Nernst Equation: E = Eo + m (ln a) Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ugarrow_forward
