
EBK LABORATORY MANUAL FOR GENERAL, ORGA
3rd Edition
ISBN: 9780321918352
Author: Timberlake
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 6.6PP
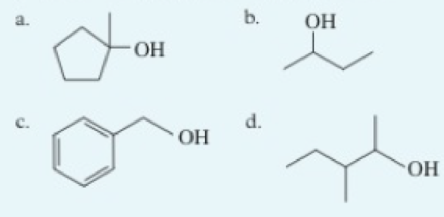
Classify each of the following alcohols as a primary (1°). secondary (2°), or tertiary (3°) alcohol:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
All these chemicals mentioned below name ways to avoid them and to clean them up if spilled?
Hazards or safety concerns associated with these chemicals:
Zinc sulfate: can cause irritation
Copper(II) sulfate: this can cause skin and eye irritation
Potassium nitrate: a fire hazard when in contact with combustible material
Silver nitrate: can cause skin, respiratory, and eye irritation such as reddish to the body.
Aluminum nitrate: thus can cause severe irritation and burns like irritation of the nose, throat, and lung with coughing, wheezing, and/or shortness of breath.
Butal
5. Draw the organic products of the following reactions.
OH
a.
HO
b.
HO
d.
OH
2-
+ CrO4
OH
+ MnO4
O
LIAIH4
OH +
NaBH4
OH
+ PCC
H₂/Pt
Please correct answer and don't use hand rating and don't use Ai solution
Chapter 6 Solutions
EBK LABORATORY MANUAL FOR GENERAL, ORGA
Ch. 6 - Prob. 6.1PPCh. 6 - Prob. 6.2PPCh. 6 - Prob. 6.3PPCh. 6 - Prob. 6.4PPCh. 6 - Prob. 6.5PPCh. 6 - Classify each of the following alcohols as a...Ch. 6 - Prob. 6.7PPCh. 6 - Prob. 6.8PPCh. 6 - Prob. 6.9PPCh. 6 - Prob. 6.10PP
Ch. 6 - Prob. 6.11PPCh. 6 - Prob. 6.12PPCh. 6 - Prob. 6.13PPCh. 6 - Prob. 6.14PPCh. 6 - Prob. 6.15PPCh. 6 - Prob. 6.16PPCh. 6 - Prob. 6.17PPCh. 6 - Prob. 6.18PPCh. 6 - Prob. 6.19PPCh. 6 - Prob. 6.20PPCh. 6 - Prob. 6.21PPCh. 6 - Prob. 6.22PPCh. 6 - When an aldehyde undergoes oxidation, the...Ch. 6 - Prob. 6.24PPCh. 6 - Prob. 6.25PPCh. 6 - Prob. 6.26PPCh. 6 - Prob. 6.27PPCh. 6 - Prob. 6.28PPCh. 6 - Prob. 6.29PPCh. 6 - Prob. 6.30PPCh. 6 - Prob. 6.31PPCh. 6 - Prob. 6.32PPCh. 6 - Prob. 6.33PPCh. 6 - Prob. 6.34PPCh. 6 - Prob. 6.35PPCh. 6 - Prob. 6.36PPCh. 6 - Prob. 6.37PPCh. 6 - Prob. 6.38PPCh. 6 - Prob. 6.39PPCh. 6 - Prob. 6.40PPCh. 6 - Prob. 6.41PPCh. 6 - Prob. 6.42PPCh. 6 - Prob. 6.43PPCh. 6 - Prob. 6.44PPCh. 6 - Prob. 6.45APCh. 6 - Prob. 6.46APCh. 6 - Prob. 6.47APCh. 6 - Prob. 6.48APCh. 6 - Prob. 6.49APCh. 6 - Prob. 6.50APCh. 6 - Prob. 6.51APCh. 6 - Prob. 6.52APCh. 6 - Prob. 6.53APCh. 6 - Classify each of the following as primary,...Ch. 6 - Prob. 6.55APCh. 6 - Prob. 6.56APCh. 6 - Prob. 6.57APCh. 6 - Prob. 6.58APCh. 6 - Prob. 6.59APCh. 6 - Prob. 6.60APCh. 6 - Prob. 6.61APCh. 6 - Prob. 6.62APCh. 6 - Prob. 6.63APCh. 6 - Prob. 6.64APCh. 6 - Prob. 6.65APCh. 6 - Prob. 6.66APCh. 6 - Prob. 6.67APCh. 6 - Prob. 6.68APCh. 6 - Prob. 6.69APCh. 6 - Draw the product of the following 1 4...Ch. 6 - Prob. 6.71APCh. 6 - Prob. 6.72APCh. 6 - Prob. 6.73APCh. 6 - Prob. 6.74APCh. 6 - Prob. 6.75APCh. 6 - Prob. 6.76APCh. 6 - Prob. 6.77CPCh. 6 - Prob. 6.78CPCh. 6 - Prob. 6.79CPCh. 6 - Prob. 6.80CPCh. 6 - How much energy is produced if a person eats 50 g...Ch. 6 - Prob. 6.82CPCh. 6 - Prob. 1IA.1QCh. 6 - Prob. 1IA.2QCh. 6 - Prob. 1IA.3QCh. 6 - Prob. 1IA.4QCh. 6 - Prob. 1IA.5QCh. 6 - Prob. 1IA.6QCh. 6 - Prob. 1IA.7QCh. 6 - Prob. 1IA.8QCh. 6 - Prob. 1IA.9QCh. 6 - Prob. 2IA.1QCh. 6 - Which oxygen n the hemiacetal product in Figure 1...Ch. 6 - Prob. 2IA.3QCh. 6 - Prob. 2IA.4QCh. 6 - Where did you place the OH for C1 (top or bottom)?Ch. 6 - Prob. 2IA.6QCh. 6 - Prob. 2IA.7QCh. 6 - Prob. 1ICCh. 6 - Prob. 2ICCh. 6 - Prob. 3ICCh. 6 - Prob. 4ICCh. 6 - Prob. 5IC
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ferrichrome is a cyclic hexa-peptide that forms a complex with iron atoms. It is a siderophore composed of three glycine and three modified ornithine residues with hydroxamate groups [-N(OH)C(=O)C-]. The 6 oxygen atoms from the three hydroxamate groups bind Fe(III) in near perfect octahedral coordination. Why does the Fe3+* prefer to bind to the hydroxamate groups? HN NH OH HN NH 'N' OH OHarrow_forwardWhat mass, in grams, of sodium chloride is needed to prepare 5.0 mL of 10.%(m/v) NaCl solution? Use dimensional analysis.arrow_forwardA 25.00mL sapmple of barium hydroxide requires 23.55mL of 0.2550 M hydrochloric acid to reach the titration end point. Calculate the molar concentration of the barium hydroxide solution. 2HCl(aq) + Ba(OH)2(aq) to BaCl2(aq) + 2H2O(l)arrow_forward
- Daily Problem #35 (Extra Credit!) Fill in the reagents. Br Br า Darrow_forwardUsing the envelope depiction we presented in class, draw out chemical structures for the following oligonucleotides. You can abbreviate the bases as Ade, Cyt, Gua, Thy, Ura.A. d(GACA)B. p(d(TATA))C. GUCUparrow_forwardthe cyclization of a farnesyl pyrophosphate to give epi-cedrol, A. Provide a stepwise mechanism for the formation of nerolidyl pyrophosphate from farnesylpyrophosphate B. Provide a stepwise mechanism for the formation of carbocation 1 from nerolidyl pyrophosphate. Number the backbone carbons of nerolidyl pyrophosphate from 1 to 11 as shown, and include the carbon numbering in your structure of 1 C. Following from B, give an arrow-pushing mechanism to convert 1 to 2 and 2 to 3. Use the backbone carbon numbering from 1 to indicate where carbon atoms ended up in 2 and 3 D. In addition to forming epi-cedrol, carbocation 3 gives three minor byproducts: a diastereomeric alcohol and two alkenes. Draw mechanisms that could give rise to these three productsarrow_forward
- Lequel des composés ci-dessous pourrait être utilisé comme réactif de départ dans la réaction suivante?arrow_forward(please correct answer and don't use hand rating) Lequel des composés ci-dessous pourrait être utilisé comme réactif de départ dans la réaction suivante?arrow_forwardPlease correct answer and don't use hand ratingarrow_forward
- Calculate the volume of 0.2445 M NaOH solution required to give 0.825g of NaOH. Use dimensional analysis.arrow_forwardIron carbonyl can be made by the direct reaction of iron metal and carbon monoxide. Fe(s) + 5 CO(g) → Fe(CO)5(s) What is the theoretical yield of Fe(CO)5 if 3.52-g of iron (Fe) istreated with CO gas having a pressure of 732-torr in a 5.50-L flask at 23 deC?arrow_forwardConsider the two isoprenoid precursors IPP and DMAPP.A. Which one is more susceptible to SN1 reaction? Explain your answer B. Which is a more likely nucleophile? Explain your answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY