
Concept explainers
(a)
Interpretation:
The given compound should be prepared using
Concept Introduction:
Addition of
Catalytic hydrogenation in presence of
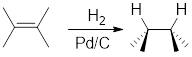
For cyclic reactants, the addition of
Enantiomers: they are chiral molecules whose mirror images are not superimposable.
Enantiomers are given configuration as R or S based on the atoms bonded with chiral (carbon bonded with four different substituents) carbon.
Carbocation: carbon ion that bears a positive charge on it.
Carbocation stability order:

Nucleophile: It donates pair of electrons to positively charged substrate resulting in the formation of
Electrophile: It is positively charged species which seeks for negative charge and hence accepts pair of electrons from negatively charged species (Nucleophiles) which results in the formation of chemical bond.
(b)
Interpretation:
The given compound should be prepared using alkene and other reagents.
Concept Introduction:
Addition of
Catalytic hydrogenation in presence of
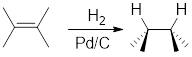
For cyclic reactants, the addition of
Enantiomers: they are chiral molecules whose mirror images are not superimposable.
Enantiomers are given configuration as R or S based on the atoms bonded with chiral (carbon bonded with four different substituents) carbon.
Carbocation: carbon ion that bears a positive charge on it.
Carbocation stability order:

Nucleophile: It donates pair of electrons to positively charged substrate resulting in the formation of chemical bond.
Electrophile: It is positively charged species which seeks for negative charge and hence accepts pair of electrons from negatively charged species (Nucleophiles) which results in the formation of chemical bond.
(c)
Interpretation:
The given compound should be prepared using alkene and other reagents.
Concept Introduction:
Addition of hydrogen halides:
In the addition of hydrogen halides over alkenes, first the
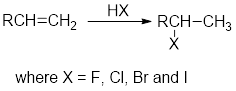
Carbocation: carbon ion that bears a positive charge on it.
Carbocation stability order:

Nucleophile: It donates pair of electrons to positively charged substrate resulting in the formation of chemical bond.
Electrophile: It is positively charged species which seeks for negative charge and hence accepts pair of electrons from negatively charged species (Nucleophiles) which results in the formation of chemical bond.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry (3rd Edition)
- Indicate whether the product of the reaction between Naphthalene and CrO3 in acetic acid at 25ºC is 1,4 naphthoquinone or phthalic anhydride.arrow_forwardIndicate the products of the reaction between CH3COCH2COOC2H5 and Na+-OC2H5.arrow_forwardPrimary, Secondary, and Tertiary Alcohols O-H O-H O-H R₁-C-H R₁-C-H R₁-C-R₁ H R₂ R₂ Primary Alcohol Secondary Alcohol ChemistryLearner.com R stands for Carbon group like ethyl methyl propyl Tertiary Alcohol If 1 carbon group with two H attached to alcoholic carbon, then primary If 2 carbon group and 1 H are attached to alcoholic carbon, then secondary IF 3 carbon group and no H attach to alcoholic carbon then tertiary. The bottom line Starting "Weak" oxidant material PCC, DMP, Swern, etc Primary alcohol Aldehyde OH Secondary alcohol Ketone OH "Strong" oxidant KMnO4, H₂CrO4 (or equivalent) OH Carboxylic acid 요 Ketone No reaction No reaction Tertiary alcohol 1. Is ethanol a primary, secondary, or tertiary alcohol? Write out the structures of ethanol and any oxidation products of ethanol. If there is more than one oxidation product, give the structure of each of the products. 2. Is 2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-propanol and any…arrow_forward
- Complete the following equations hand written pleasearrow_forwardComplete the following equations please hand written pleasearrow_forwardUsing the Nernst equation to calculate nonstandard cell voltage A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 3+ 3Cu²+ (aq) +2Al(s) → 3 Cu(s)+2A1³* (aq) 2+ Suppose the cell is prepared with 5.29 M Cu in one half-cell and 2.49 M A1³+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. x10 μ ☑ 00. 18 Ar Иarrow_forward
- Please help me solve this homework problemarrow_forwardPlease help me answer this homework questionarrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3+ H2(g)+2OH¯ (aq) + 2Fe³+ (aq) → 2H₂O (1)+2Fe²+ (aq) 0 kJ x10 Х ? olo 18 Ararrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

