
Thermodynamics: An Engineering Approach
8th Edition
ISBN: 9780073398174
Author: Yunus A. Cengel Dr., Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 5.5, Problem 86P
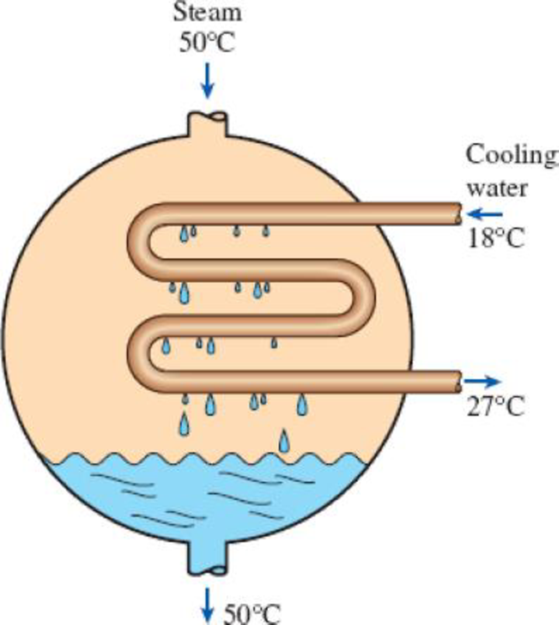
Steam is to be condensed in the condenser of a steam power plant at a temperature of 50°C with cooling water from a nearby lake, which enters the tubes of the condenser at 18°C at a rate of 101 kg/s and leaves at 27°C. Determine the rate of condensation of the steam in the condenser.
FIGURE P5–86
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Important:
I've posted this question twice and received incorrect answers. I've clearly stated that I don't require AI-generated working out. I need a genuine, expert-written solution with proper working. If you can't provide that, refer this question to someone who can please!.
Note:
Please provide a clear, step-by-step handwritten solution (no AI involvement). I require an expert-level answer and will assess it based on quality and accuracy with that I'll give it a thumbs up or down!. Hence, refer to the provided image for clarity. Double-check everything for correctness before submitting. Thank you!
Note:
Please provide a clear, step-by-step simplified handwritten working out (no explanations!), ensuring it is done without any AI involvement. I require an expert-level answer, and I will assess and rate based on the quality and accuracy of your work and refer to the provided image for more clarity. Make sure to double-check everything for correctness before submitting appreciate your time and effort!.
Question:
Note:
Please provide a clear, step-by-step simplified handwritten working out (no explanations!), ensuring it is done without any AI involvement. I require an expert-level answer, and I will assess and rate based on the quality and accuracy of your work and refer to the provided image for more clarity. Make sure to double-check everything for correctness before submitting appreciate your time and effort!.
Question:
If the flow rate through the system below is 0.04m3s-1, find the difference in elevation H of the two reservoirs.
Chapter 5 Solutions
Thermodynamics: An Engineering Approach
Ch. 5.5 - Prob. 1PCh. 5.5 - Define mass and volume flow rates. How are they...Ch. 5.5 - Does the amount of mass entering a control volume...Ch. 5.5 - Consider a device with one inlet and one outlet....Ch. 5.5 - The ventilating fan of the bathroom of a building...Ch. 5.5 - 5–6E Air whose density is 0.078 lbm/ft3 enters the...Ch. 5.5 - 5–7 Air enters a 28-cm diameter pipe steadily at...Ch. 5.5 - A steady-flow compressor is used to compress...Ch. 5.5 - A 2-m3 rigid tank initially contains air whose...Ch. 5.5 - 5–10 A cyclone separator like that in Fig. P5–10...
Ch. 5.5 - 5–11 A spherical hot-air balloon is initially...Ch. 5.5 - A desktop computer is to be cooled by a fan whose...Ch. 5.5 - 5–13 A pump increases the water pressure from 100...Ch. 5.5 - Refrigerant-134a enters a 28-cm-diameter pipe...Ch. 5.5 - Prob. 15PCh. 5.5 - Prob. 16PCh. 5.5 - 5–17C What is flow energy? Do fluids at rest...Ch. 5.5 - How do the energies of a flowing fluid and a fluid...Ch. 5.5 - Prob. 19PCh. 5.5 - Prob. 20PCh. 5.5 - Refrigerant-134a enters the compressor of a...Ch. 5.5 - Steam is leaving a pressure cooker whose operating...Ch. 5.5 - A diffuser is an adiabatic device that decreases...Ch. 5.5 - The kinetic energy of a fluid increases as it is...Ch. 5.5 - Prob. 25PCh. 5.5 - Air enters a nozzle steadily at 50 psia, 140F, and...Ch. 5.5 - The stators in a gas turbine are designed to...Ch. 5.5 - The diffuser in a jet engine is designed to...Ch. 5.5 - Air at 600 kPa and 500 K enters an adiabatic...Ch. 5.5 - Prob. 30PCh. 5.5 - Prob. 31PCh. 5.5 - Air at 13 psia and 65F enters an adiabatic...Ch. 5.5 - Carbon dioxide enters an adiabatic nozzle steadily...Ch. 5.5 - Refrigerant-134a at 700 kPa and 120C enters an...Ch. 5.5 - Prob. 35PCh. 5.5 - Refrigerant-134a enters a diffuser steadily as...Ch. 5.5 - Prob. 38PCh. 5.5 - Air at 80 kPa, 27C, and 220 m/s enters a diffuser...Ch. 5.5 - 5–40C Consider an air compressor operating...Ch. 5.5 - Prob. 41PCh. 5.5 - Somebody proposes the following system to cool a...Ch. 5.5 - 5–43E Air flows steadily through an adiabatic...Ch. 5.5 - Prob. 44PCh. 5.5 - Prob. 45PCh. 5.5 - Steam flows steadily through an adiabatic turbine....Ch. 5.5 - Prob. 48PCh. 5.5 - Steam flows steadily through a turbine at a rate...Ch. 5.5 - Prob. 50PCh. 5.5 - Carbon dioxide enters an adiabatic compressor at...Ch. 5.5 - Prob. 52PCh. 5.5 - 5–54 An adiabatic gas turbine expands air at 1300...Ch. 5.5 - Prob. 55PCh. 5.5 - Prob. 56PCh. 5.5 - Air enters the compressor of a gas-turbine plant...Ch. 5.5 - Why are throttling devices commonly used in...Ch. 5.5 - Would you expect the temperature of air to drop as...Ch. 5.5 - Prob. 60PCh. 5.5 - During a throttling process, the temperature of a...Ch. 5.5 - Refrigerant-134a is throttled from the saturated...Ch. 5.5 - A saturated liquidvapor mixture of water, called...Ch. 5.5 - Prob. 64PCh. 5.5 - A well-insulated valve is used to throttle steam...Ch. 5.5 - Refrigerant-134a enters the expansion valve of a...Ch. 5.5 - Prob. 68PCh. 5.5 - Consider a steady-flow heat exchanger involving...Ch. 5.5 - Prob. 70PCh. 5.5 - Prob. 71PCh. 5.5 - Prob. 72PCh. 5.5 - Prob. 73PCh. 5.5 - Prob. 74PCh. 5.5 - Prob. 76PCh. 5.5 - Steam is to be condensed on the shell side of a...Ch. 5.5 - Prob. 78PCh. 5.5 - Air (cp = 1.005 kJ/kgC) is to be preheated by hot...Ch. 5.5 - Prob. 80PCh. 5.5 - Refrigerant-134a at 1 MPa and 90C is to be cooled...Ch. 5.5 - Prob. 82PCh. 5.5 - An air-conditioning system involves the mixing of...Ch. 5.5 - The evaporator of a refrigeration cycle is...Ch. 5.5 - Steam is to be condensed in the condenser of a...Ch. 5.5 - Steam is to be condensed in the condenser of a...Ch. 5.5 - Two mass streams of the same ideal gas are mixed...Ch. 5.5 - Prob. 89PCh. 5.5 - A 110-volt electrical heater is used to warm 0.3...Ch. 5.5 - The fan on a personal computer draws 0.3 ft3/s of...Ch. 5.5 - Prob. 92PCh. 5.5 - 5–93 A scaled electronic box is to be cooled by...Ch. 5.5 - Prob. 94PCh. 5.5 - Prob. 95PCh. 5.5 - Prob. 96PCh. 5.5 - Prob. 97PCh. 5.5 - A computer cooled by a fan contains eight PCBs,...Ch. 5.5 - Prob. 99PCh. 5.5 - A long roll of 2-m-wide and 0.5-cm-thick 1-Mn...Ch. 5.5 - Prob. 101PCh. 5.5 - Prob. 102PCh. 5.5 - A house has an electric heating system that...Ch. 5.5 - Steam enters a long, horizontal pipe with an inlet...Ch. 5.5 - Refrigerant-134a enters the condenser of a...Ch. 5.5 - Prob. 106PCh. 5.5 - Water is heated in an insulated, constant-diameter...Ch. 5.5 - Prob. 108PCh. 5.5 - Air enters the duct of an air-conditioning system...Ch. 5.5 - A rigid, insulated tank that is initially...Ch. 5.5 - 5–113 A rigid, insulated tank that is initially...Ch. 5.5 - Prob. 114PCh. 5.5 - A 0.2-m3 rigid tank equipped with a pressure...Ch. 5.5 - Prob. 116PCh. 5.5 - Prob. 117PCh. 5.5 - Prob. 118PCh. 5.5 - Prob. 119PCh. 5.5 - An air-conditioning system is to be filled from a...Ch. 5.5 - Oxygen is supplied to a medical facility from ten...Ch. 5.5 - Prob. 122PCh. 5.5 - A 0.3-m3 rigid tank is filled with saturated...Ch. 5.5 - Prob. 124PCh. 5.5 - Prob. 125PCh. 5.5 - Prob. 126PCh. 5.5 - The air-release flap on a hot-air balloon is used...Ch. 5.5 - An insulated 0.15-m3 tank contains helium at 3 MPa...Ch. 5.5 - An insulated 40-ft3 rigid tank contains air at 50...Ch. 5.5 - A vertical pistoncylinder device initially...Ch. 5.5 - A vertical piston-cylinder device initially...Ch. 5.5 - Prob. 135RPCh. 5.5 - Prob. 136RPCh. 5.5 - Air at 4.18 kg/m3 enters a nozzle that has an...Ch. 5.5 - An air compressor compresses 15 L/s of air at 120...Ch. 5.5 - 5–139 Saturated refrigerant-134a vapor at 34°C is...Ch. 5.5 - A steam turbine operates with 1.6 MPa and 350C...Ch. 5.5 - Prob. 141RPCh. 5.5 - Prob. 142RPCh. 5.5 - Prob. 143RPCh. 5.5 - Steam enters a nozzle with a low velocity at 150C...Ch. 5.5 - Prob. 146RPCh. 5.5 - Prob. 147RPCh. 5.5 - Prob. 148RPCh. 5.5 - Prob. 149RPCh. 5.5 - Cold water enters a steam generator at 20C and...Ch. 5.5 - Prob. 151RPCh. 5.5 - An ideal gas expands in an adiabatic turbine from...Ch. 5.5 - Prob. 153RPCh. 5.5 - Prob. 154RPCh. 5.5 - Prob. 155RPCh. 5.5 - Prob. 156RPCh. 5.5 - Prob. 157RPCh. 5.5 - Prob. 158RPCh. 5.5 - Prob. 159RPCh. 5.5 - Prob. 160RPCh. 5.5 - Prob. 161RPCh. 5.5 - Prob. 162RPCh. 5.5 - Prob. 163RPCh. 5.5 - The ventilating fan of the bathroom of a building...Ch. 5.5 - Determine the rate of sensible heat loss from a...Ch. 5.5 - An air-conditioning system requires airflow at the...Ch. 5.5 - The maximum flow rate of standard shower heads is...Ch. 5.5 - An adiabatic air compressor is to be powered by a...Ch. 5.5 - Prob. 171RPCh. 5.5 - Prob. 172RPCh. 5.5 - Prob. 173RPCh. 5.5 - Prob. 174RPCh. 5.5 - Prob. 175RPCh. 5.5 - A tank with an internal volume of 1 m3 contains...Ch. 5.5 - A liquid R-134a bottle has an internal volume of...Ch. 5.5 - Prob. 179RPCh. 5.5 - Prob. 181RPCh. 5.5 - Prob. 182RPCh. 5.5 - Prob. 184RPCh. 5.5 - A pistoncylinder device initially contains 1.2 kg...Ch. 5.5 - In a single-flash geothermal power plant,...Ch. 5.5 - The turbocharger of an internal combustion engine...Ch. 5.5 - A building with an internal volume of 400 m3 is to...Ch. 5.5 - Prob. 189RPCh. 5.5 - Prob. 190RPCh. 5.5 - Prob. 191RPCh. 5.5 - Prob. 192FEPCh. 5.5 - Prob. 193FEPCh. 5.5 - An adiabatic heat exchanger is used to heat cold...Ch. 5.5 - A heat exchanger is used to heat cold water at 15C...Ch. 5.5 - An adiabatic heat exchanger is used to heat cold...Ch. 5.5 - In a shower, cold water at 10C flowing at a rate...Ch. 5.5 - Prob. 198FEPCh. 5.5 - Hot combustion gases (assumed to have the...Ch. 5.5 - Steam expands in a turbine from 4 MPa and 500C to...Ch. 5.5 - Steam is compressed by an adiabatic compressor...Ch. 5.5 - Refrigerant-134a is compressed by a compressor...Ch. 5.5 - Prob. 203FEPCh. 5.5 - Prob. 204FEPCh. 5.5 - Air at 27C and 5 atm is throttled by a valve to 1...Ch. 5.5 - Steam at 1 MPa and 300C is throttled adiabatically...Ch. 5.5 - Air is to be heated steadily by an 8-kW electric...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Note: Please provide a clear, step-by-step simplified handwritten working out (no explanations!), ensuring it is done without any AI involvement. I require an expert-level answer, and I will assess and rate based on the quality and accuracy of your work and refer to the provided image for more clarity. Make sure to double-check everything for correctness before submitting thanks!. Question: (In the image as provided)arrow_forwardNote: Please provide a clear, step-by-step simplified handwritten working out (no explanations!), ensuring it is done without any AI involvement. I require an expert-level answer, and I will assess and rate based on the quality and accuracy of your work and refer to the provided image for more clarity. Make sure to double-check everything for correctness before submitting thanks!. Question: The rectangular gate shown below is 3 m wide. Compute the force P needed to hold the gate in the position shown.arrow_forwardNote: Please provide a clear, step-by-step simplified handwritten working out (no explanations!), ensuring it is done without any AI involvement. I require an expert-level answer, and I will assess and rate based on the quality and accuracy of your work and refer to the provided image for more clarity. Make sure to double-check everything for correctness before submitting thanks!. Question1: If the following container is 0.6m high, 1.2m wide and half full with water, determine the pressure acting at points A, B, and C if ax=2.6ms^-2.arrow_forward
- Please read the imagearrow_forwardChapter 12 - Lecture Notes.pptx: (MAE 272-01) (SP25) DY... Scoresarrow_forwardConsider a large 6-cm-thick stainless steel plate (k = 15.1 W/m-K) in which heat is generated uniformly at a rate of 5 × 105 W/m³. Both sides of the plate are exposed to an environment at 30°C with a heat transfer coefficient of 60 W/m²K. Determine the value of the highest and lowest temperature. The highest temperature is The lowest temperature is °C. °C.arrow_forwardSketch and explain a PV Diagram and a Temperature Entropy Diagram for a 4 stroke diesel engine please, please explain into detail the difference bewteen the two and referance the a diagram. Please include a sketch or an image of each diagramarrow_forwardDraw left view of the first orthographic projectionarrow_forwardSketch and Describe a timing diagram for a 2 stroke diesel engine emphasis on the 2 stroke as my last answer explained 4 stroke please include a diagram or sketch.arrow_forwardA 4 ft 200 Ib 1000 Ib.ft C 2 ft 350 Ib - за в 2.5 ft 150 Ib 250 Ib 375 300 Ib Replace the force system acting on the frame. shown in the figure by a resultant force (magnitude and direction), and specify where its line of action intersects member (AB), measured from point (A).arrow_forwardA continuous flow calorimeter was used to obtain the calorific value of a sample of fuel and the following data collected: Mass of fuel: 2.25 kgInlet water temperature: 11 ° COutlet water temperature 60 ° CQuantity of water: 360 Liters Calorimeter efficiency: 85%Calculate the calorific value of the sample ( kJ / kg ). ive submitted this question twice and have gotten two way different answers. looking for some help thanksarrow_forward15 kg of steel ball bearings at 100 ° C is immersed in 25 kg of water at 20 ° C . Assuming no loss of heat to or from the container, calculate the final temperature of the water after equilibrium has been attained.Specific heat of steel: 0.4857 kJ / kg / ° KSpecific heat of water: 4.187 kJ / kg / ° Karrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Power Plant Explained | Working Principles; Author: RealPars;https://www.youtube.com/watch?v=HGVDu1z5YQ8;License: Standard YouTube License, CC-BY