
Concept explainers
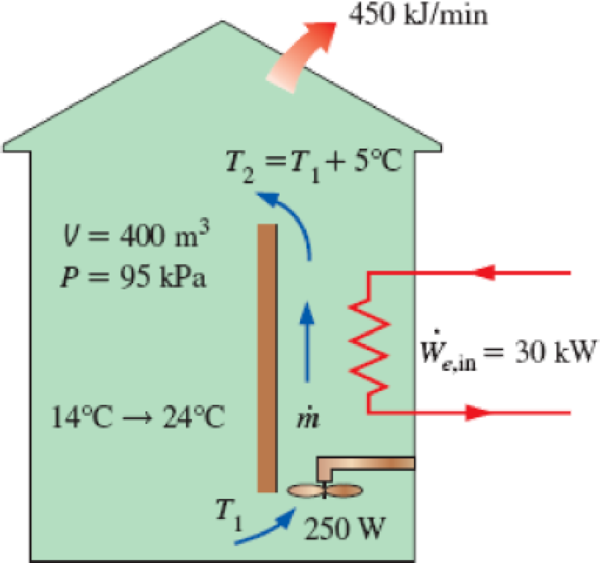
A building with an internal volume of 400 m3 is to be heated by a 30-kW electric resistance heater placed in the duct inside the building. Initially, the air in the building is at 14°C, and the local atmospheric pressure is 95 kPa. The building is losing heat to the surroundings at a steady rate of 450 kJ/min. Air is forced to flow through the duct and the heater steadily by a 250-W fan, and it experiences a temperature rise of 5°C each time it passes through the duct, which may be assumed to be adiabatic.
- (a) How long will it take for the air inside the building to reach an average temperature of 24°C?
- (b) Determine the average mass flow rate of air through the duct.
FIGURE P5–173
(a)
The time taken to attain the building’s average temperature of
Answer to Problem 188RP
The time taken to attain the building’s average temperature of
Explanation of Solution
Consider the entire building as system and the air circulates the in the building itself. There is no leakage to the surrounding.
The air flows at steady state through one inlet and one exit system (pipe and duct flow). Hence, the inlet and exit mass flow rates are equal.
Write the energy balance equation.
Here, the heat transfer is
In this system two work inputs are involved namely, the work input to the electric heater
The Equations (I) reduced as follows.
Here, there is no mass leakage from the building to the surrounding. The mass of air circulates in the building itself. Hence, inlet and exit enthalpies are neglected.
The change in internal energy is expresses as follow.
Here, the specific heat at constant volume is
Neglect the inlet and exit enthalpies and substitute
Equation (II).
Express the Equation (III) with respect to change of time and rearrange it to obtain
Write the formula for mass of air
The mass flow rate
Here, the change in time or time interval is
Refer Table A-1, “Molar mass, gas constant, and critical-point properties”.
The gas constant of air
Refer Table A-2, “Ideal-gas specific heats of various common gases”.
The specific heat at constant volume
Conclusion:
Substitute
Substitute
Substitute
Thus, the time taken to attain the building’s average temperature of
(b)
The average mass flow rate of air through the duct.
Answer to Problem 188RP
The average mass flow rate of air through the duct is
Explanation of Solution
Consider the heating duct with fan and heater only as the system. The air passes through in it steadily.
The system is at steady state. Hence, the rate of change in net energy of the system becomes zero.
The heating duct is an adiabatic duct. Hence, there is no heat loss.
The Equations (II) reduced as follows.
Express the Equation (VII) with respect to change of time as follows.
The change in enthalpy is expresses as follow.
Here, the specific heat at constant pressure is
Substitute
Refer Table A-2, “Ideal-gas specific heats of various common gases”.
The specific heat at constant pressure
Conclusion:
It is given that the temperature rise is
Substitute
Thus, The average mass flow rate of air through the duct is
Want to see more full solutions like this?
Chapter 5 Solutions
Thermodynamics: An Engineering Approach
- The ventilating fan of the bathroom of a building has a volume flow rate of 30 L/s and runs continuously. The building is located in San Francisco, California, where the average winter temperature is 12.2°C, and it is maintained at 22°C at all times. The building is heated by electricity whose unit cost is $0.12/kWh. Determine the amount and cost of the heat “vented out” per month in winterarrow_forwardConsider a well-insulated piston-cylinder assembly. The volume of the cylinder is 3.258 m3. You can assume the piston to be frictionless and that it does not occupy a significant volume in the cylinder. Initially the piston is placed such that the entire volume of the cylinder is filled with steam at 100 kPa and 200°C. The cylinder is connected to a pipeline carrying air at 500 kPa and 250°C. The valve between the pipeline and the cylinder is opened slightly allowing air to enter the cylinder very slowly until the pressure in the cylinder reaches 500 kPa. The valve is then turned off. Assume air behaves like an ideal gas with a constant heat capacity of Cp=7R/2. a) What will be the final temperature of the air if the piston is nonconducting? (Note: steam is being compressed adiabatically and very slowly by means of a frictionless piston) b) Suppose the insulation pad at the bottom of the cylinder is removed and heat is transferred to the steam side to keep its temperature constant at…arrow_forwardA cooling device removes the heat in the amount of 2368 J/s from the cooled environment at -2°C and throws it into the environment at 28°C. What is the minimum amount of work that must be submitted?arrow_forward
- A piston-cylinder device contains 0.1 m3 of refrigerant 134a at 0.24 MPa and 40ºC. Initially the piston is fixed with a pin. Heat is transferred now to the refrigerant from a source at 100ºC until the pressure rises to 0.28 MPa. Then, heat is given to an environment with a temperature of 25ºC at a constant pressure (the pin is pulled and in this case, the mass of the piston and the masses on it and the pressure created by the atmospheric pressure are equal to the pressure inside the cylinder) the temperature is brought to a temperature of 50ºC. a) Determine the heat and work interaction for each process b) Sketch the P-v and T-s diagrams of the processes with respect to the saturation lines c) Determine the entropy change of the refrigerant 134a during these two processes d) Determine the entropy generation during these processes. Are these processes appropriate to 2. law of thermodynamics, explainarrow_forwardDetermine the rate of heat released (in kW) by the steam with 88.0% quality thatenters the evaporator at 1800 kg/h at 132C and exits as a subcooled liquid at 100C.Assume that the condensate outlet pressure is the same as the steam inlet pressure.The specific heat of liquid water is 4.187 kJ/kg-Carrow_forwardA passive solar house that is losing heat to the outdoors at an average rate of 50,000 kJ/h is maintained at 22°C at all times during a winter night for 10 h. The house is to be heated by 50 glass containers each containing 20 L of water that is heated to 80°C during the day by absorbing solar energy. A thermostat-controlled 15-kW back-up electric resistance heater turns on whenever necessary to keep the house at 22°C. (a) How long did the electric heating system run that night? (b) How long would the electric heater run that night if the house incorporated no solar heating?arrow_forward
- Water is being heated in a close pan on top of a range while being stirred by a paddle wheel. During the process, 30kj of heat are transferred to the water, and 5kj of heat is lost to the surrounding air. The paddle-wheel work amounts to 500 N*m. Determine the final energy of the system if its initial energy is 10kj.arrow_forwardA passive solar house that is losing heat to the outdoors at an average rate of 50,000 kJ/h is maintained at 22°C at all times during a winter night for 10 h. The house is to be heated by 50 glass containers each containing 20 L of water that is heated to 80°C during the day by absorbing solar energy. A thermostat-controlled 15-kW backup electric resistance heater turns on whenever necessary to keep the house at 22°C. How long did the electric heating system run that night?arrow_forwardA variable-load piston-cylinder device contains air (cp = 1.005 kJ/kgK; cv = 0.718 kJ/kgK) at 500 kPa and T=12 oC. A paddle wheelequipped within the system and turned by an external electric motor until 65 kJ/kg of work has been transferred to the air. During this process the gas volume is quadrupled while maintaining the temperature constant by transferring heat to the gas. Determine (a) the final pressure, (b) the amount of required heattransfer (c) Show this process on a P-v diagram. Do not use Table A-17 while solving this problem. YOUR ANSWER SHEET SHOULD INCLUDE THE SOLUTION AND THE TABLE BELOW (a) Pinal [kPa] = (b) q [kJ/kg]arrow_forward
- A 5-ft3 rigid tank initially contains refrigerant-134a at 60 psia and 100 percent quality. The tank is connected by a valve to a supply line that carries refrigerant-134a at 140 psia and 80°F. The valve is now opened, allowing the refrigerant to enter the tank, and is closed when it is observed that the tank contains only saturated liquid at 100 psia. Determine the amount of heat transfer with the surroundings at 70°F.arrow_forwardIt is common knowledge that the temperature of air rises as it is compressed. An inventor thought about using this high-temperature air to heat buildings. He used a compressor driven by an electric motor. The inventor claims that the compressed hot-air system is 25 percent more efficient than a resistance heating system that provides an equivalent amount of heating. Is this claim valid, or is this just another perpetualmotion machine? Explain.arrow_forwardA 2.4-m-high 200-m2 house is maintained at 22°C by an air-conditioning system whose COP is 3.2. It is estimated that the kitchen, bath, and other ventilating fans of the house discharge a houseful of conditioned air once every hour. If the average outdoor temperature is 32°C, the density of air is 1.20 kg/m3 , and the unit cost of electricity is $0.10/kWh, the amount of money “vented out” by the fans in 10 hours is (a) $0.50 (b) $1.6 (c) $5.0 (d) $11.0 (e) $16.0arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





