
A piston–cylinder device initially contains 1.2 kg of air at 700 kPa and 200°C. At this state, the piston is touching on a pair of stops. The mass of the piston is such that 600-kPa pressure is required to move it. A valve at the bottom of the tank is opened, and air is withdrawn from the cylinder. The valve is closed when the volume of the cylinder decreases to 80 percent of the initial volume. If it is estimated that 40 kJ of heat is lost from the cylinder, determine (a) the final temperature of the air in the cylinder, (b) the amount of mass that has escaped from the cylinder, and (c) the work done. Use constant specific heats at the average temperature.
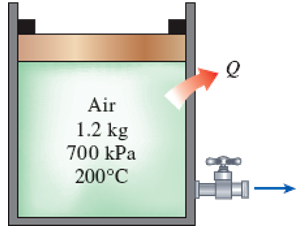
FIGURE P5–183
(a)
The final temperature of air in the cylinder.
Answer to Problem 185RP
The final temperature of air in the cylinder is
Explanation of Solution
Write the equation of mass balance.
Here, the inlet mass is
The change in mass of the system for the control volume is expressed as,
Here, the suffixes 1 and 2 indicates the initial and final states of the system.
Consider the piston-cylinder as the control volume. Initially the cylinder is filled with air and the valve is in closed position, further no other mass is allowed to enter the cylinder. Hence, the inlet mass is neglected i.e.
Rewrite the Equation (I) as follows.
Write the formula for initial volume of air present in the cylinder.
Here, the mass of air is
Write the formula for mass of air present in the cylinder at final state.
Here, the subscript 2 indicates the final state.
Write the energy balance equation.
Here, the heat transfer is
The pressure of
The Equation (V) reduced as follows.
Write the formula for boundary work done on the cylinder.
Here, the pressure required to move the piston is
The enthalpy and internal energy in terms of temperature and specific heats are expressed as follows.
Rewrite the Equation (VI) as follows.
The temperature of the air while exiting the cylinder is considered as the average temperature of initial and final temperatures.
Refer Table A-1, “Molar mass, gas constant, and critical-point properties”.
The gas constant
Refer Table A-2b, “Ideal-gas specific heats of various common gases”.
The specific heat at constant pressure
Conclusion:
Substitute
It is given that the final volume is 80 % of initial volume.
Substitute
Substitute
Substitute
Substitute
Use Engineering Equation Solver (EES) or online calculator to solve the Equation (X) and obtain the value of
Thus, the final temperature of air in the cylinder is
(b)
The amount of mass escaped from the cylinder.
Answer to Problem 185RP
The amount of mass escaped from the cylinder is
Explanation of Solution
The amount of mass escaped from the cylinder is nothing but the mass of air vented out until final state i.e.
Refer Equation (II) and (IX).
Conclusion:
Substitute
Thus, the amount of mass escaped from the cylinder is
(c)
The work done.
Answer to Problem 185RP
The amount of mass escaped from the cylinder is
Explanation of Solution
The work done is nothing but the work done on the piston to move it i.e. boundary work
Refer part (a).
Thus, the work done is
Want to see more full solutions like this?
Chapter 5 Solutions
Thermodynamics: An Engineering Approach
- A frictionless piston cylinder device contains 3 L of saturated liquid water at a temperature of 110°C. Water is stirred by a paddle wheel for 22 minutes while a current of 7.5 A flows through a resistor placed in the water. If the 74% of the water remains at the liquid phase and the rest water is evaporated during this constant pressure process. If the voltage of the electricity source is 160 volts, determine (a) the initial pressure and (b) the amount of work added to the water by the paddle wheel. Also, show the process on a P-v diagram with respect to saturation lines. H2O P= constant We sharrow_forwardIn this example, water is contained in a rigid container with a tight fitting lid. With a pressure of 700 kPa, a mass of 1.78 kg of saturated liquid, and a mass of 0.22 kg of saturated vapour, the saturated liquid has a mass of 1.78 kg and the saturated vapour has a mass of 0.22 kg. When the water pressure reaches 8 Mpa, more heat is added to it. Calculate the water's final internal energy. Values in between solutions should not be rounded off.arrow_forwardDetermine the quality of steam at 169.06 kPa when 270 kJ/kg of energy are lost from saturated steam. What is the steam temperature?arrow_forward
- A rigid vessel contains 5 kg of wet steam at 0.4 Mpa. After the addition of 9,585 J the steam has a pressure of 2.0 Mpa and a temperature of 700C. Determine the initial internal energy and the specific volume of the steam.arrow_forwardA frictionless piston-cylinder device contains 3 L of saturated liquid water at a pressure of 200 kPa. Water is stirred by a paddle wheel for 22 minutes while a current of 7.5 A flows through a resistor placed in the water. If the 74% of the water remains at the liquid phase and the rest water is evaporated during this constant pressure process. If the voltage of the electricity source is 160 volts, determine (a) the final temperature and (b) the amount of work added to the water by the paddle wheel. Also, show the process on a P-v diagram with respect to saturation lines. H20 P constant W.arrow_forwardA frictionless piston–cylinder device initially contains 170 L of saturated liquid refrigerant-134a. The piston is free to move, and its mass is such that it maintains a pressure of 900 kPa on the refrigerant. The refrigerant is now heated until its temperature rises to 70°C. Calculate the work done during this process.arrow_forward
- A piston-cylinder device initially contains 0.65 m3 of saturated water vapor at 225 kPa. At this state, the piston is resting on a set of stops, and the mass of the piston is such that a pressure of 300 kPa is required to move it. Heat is now slowly transferred to the steam until the volume doubles. Show the process on a P-V diagram with respect to saturation lines and determine;(a) the final temperature,(b) the work done during this process,(c) the total heat transfers.arrow_forwardA rigid tank contains 7.5 kg of saturated water mixture at 400 kPa. A valve at the bottom of the tank is now opened, and liquid is withdrawn from the tank. Heat is transferred to the steam such that the pressure inside the tank remains constant. The valve is closed when no liquid is left in the tank. If it is estimated that a total of 5 kJ of heat is transferred to the tank, determine the amount of mass that has escaped.arrow_forwardTwo closed vessel A and B contains air and is connected together by a valve. Vessel A has an unknown volume at 3500kPa and 60°C, while vessel B has a volume of 75 L at 200kPa and 20°C. After a while, the valve was opened and the resulting pressure and temperature of the mixture are 1800kPa and 32°C, respectively. Determine the volume of vessel A.arrow_forward
- A piston-cylinder device initially contains 1 kg saturated liquid water at 220°C. Now heat is transferred to the water until the volume expands quadruple of its initial volume. Eventually, the cylinder contains saturated vapor only. Determine the volume at the final stage. (i) (ii) Determine the final temperature and pressure. (iii) Determine the internal energy change of the water. (iv) Sketch the T-v diagram and include all the related information.arrow_forwardA piston–cylinder device initially contains 1.2 kg of air at 700 kPa and 200°C. At this state, the piston is touching on a pair of stops. The mass of the piston is such that 600-kPa pressure is required to move it. A valve at the bottom of the tank is opened, and air is withdrawn from the cylinder. The valve is closed when the volume of the cylinder decreases to 80 percent of the initial volume. If it is estimated that 40 kJ of heat is lost from the cylinder, determine the work done. Use constant specific heats at the average temperature.arrow_forwardA piston–cylinder device initially contains 1.2 kg of air at 700 kPa and 200°C. At this state, the piston is touching on a pair of stops. The mass of the piston is such that 600-kPa pressure is required to move it. A valve at the bottom of the tank is opened, and air is withdrawn from the cylinder. The valve is closed when the volume of the cylinder decreases to 80 percent of the initial volume. If it is estimated that 40 kJ of heat is lost from the cylinder, determine the final temperature of the air in the cylinder.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





