
Thermodynamics: An Engineering Approach
8th Edition
ISBN: 9780073398174
Author: Yunus A. Cengel Dr., Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5.5, Problem 164RP
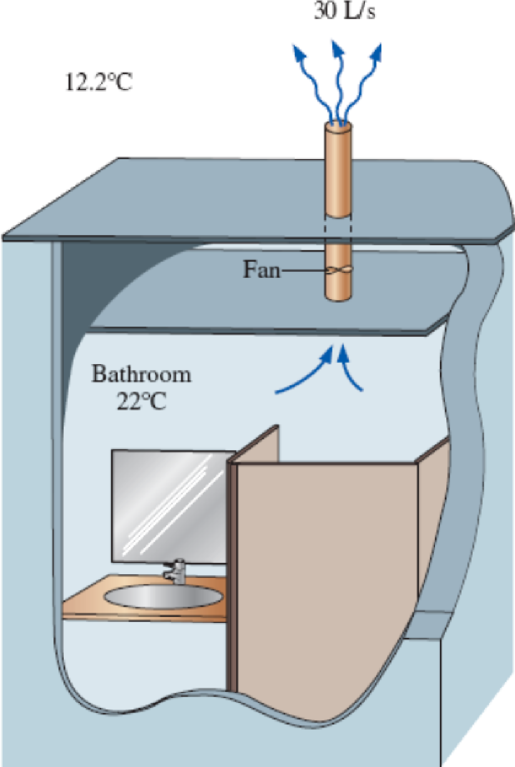
The ventilating fan of the bathroom of a building has a volume flow rate of 30 L/s and runs continuously. The building is located in San Francisco, California, where the average winter temperature is 12.2°C, and it is maintained at 22°C at all times. The building is heated by electricity whose unit cost is $0.12/kWh. Determine the amount and cost of the heat “vented out” per month in winter.
FIGURE P5–168
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
+1.
0,63 fin
r= 0.051
P
The stepped rod in sketch is subjected to a tensile
force that varies between 4000 and 7000 lb. The
rod has a machined surface finish everywhere except
the shoulder area,
where a grinding operation has
been performed to improve the fatigue resistance
of the rod. Using a 99% probability of survival,
determine the safety factor for infinite life if
the rod is made of AISI 1080 steel, quenched
and tempered at 800°c Use the Goodman line.
Does the part fail at the fillet? Explain
Solve this problem and show all of the work
Solve this problem and show all of the work
Chapter 5 Solutions
Thermodynamics: An Engineering Approach
Ch. 5.5 - Prob. 1PCh. 5.5 - Define mass and volume flow rates. How are they...Ch. 5.5 - Does the amount of mass entering a control volume...Ch. 5.5 - Consider a device with one inlet and one outlet....Ch. 5.5 - The ventilating fan of the bathroom of a building...Ch. 5.5 - 5–6E Air whose density is 0.078 lbm/ft3 enters the...Ch. 5.5 - 5–7 Air enters a 28-cm diameter pipe steadily at...Ch. 5.5 - A steady-flow compressor is used to compress...Ch. 5.5 - A 2-m3 rigid tank initially contains air whose...Ch. 5.5 - 5–10 A cyclone separator like that in Fig. P5–10...
Ch. 5.5 - 5–11 A spherical hot-air balloon is initially...Ch. 5.5 - A desktop computer is to be cooled by a fan whose...Ch. 5.5 - 5–13 A pump increases the water pressure from 100...Ch. 5.5 - Refrigerant-134a enters a 28-cm-diameter pipe...Ch. 5.5 - Prob. 15PCh. 5.5 - Prob. 16PCh. 5.5 - 5–17C What is flow energy? Do fluids at rest...Ch. 5.5 - How do the energies of a flowing fluid and a fluid...Ch. 5.5 - Prob. 19PCh. 5.5 - Prob. 20PCh. 5.5 - Refrigerant-134a enters the compressor of a...Ch. 5.5 - Steam is leaving a pressure cooker whose operating...Ch. 5.5 - A diffuser is an adiabatic device that decreases...Ch. 5.5 - The kinetic energy of a fluid increases as it is...Ch. 5.5 - Prob. 25PCh. 5.5 - Air enters a nozzle steadily at 50 psia, 140F, and...Ch. 5.5 - The stators in a gas turbine are designed to...Ch. 5.5 - The diffuser in a jet engine is designed to...Ch. 5.5 - Air at 600 kPa and 500 K enters an adiabatic...Ch. 5.5 - Prob. 30PCh. 5.5 - Prob. 31PCh. 5.5 - Air at 13 psia and 65F enters an adiabatic...Ch. 5.5 - Carbon dioxide enters an adiabatic nozzle steadily...Ch. 5.5 - Refrigerant-134a at 700 kPa and 120C enters an...Ch. 5.5 - Prob. 35PCh. 5.5 - Refrigerant-134a enters a diffuser steadily as...Ch. 5.5 - Prob. 38PCh. 5.5 - Air at 80 kPa, 27C, and 220 m/s enters a diffuser...Ch. 5.5 - 5–40C Consider an air compressor operating...Ch. 5.5 - Prob. 41PCh. 5.5 - Somebody proposes the following system to cool a...Ch. 5.5 - 5–43E Air flows steadily through an adiabatic...Ch. 5.5 - Prob. 44PCh. 5.5 - Prob. 45PCh. 5.5 - Steam flows steadily through an adiabatic turbine....Ch. 5.5 - Prob. 48PCh. 5.5 - Steam flows steadily through a turbine at a rate...Ch. 5.5 - Prob. 50PCh. 5.5 - Carbon dioxide enters an adiabatic compressor at...Ch. 5.5 - Prob. 52PCh. 5.5 - 5–54 An adiabatic gas turbine expands air at 1300...Ch. 5.5 - Prob. 55PCh. 5.5 - Prob. 56PCh. 5.5 - Air enters the compressor of a gas-turbine plant...Ch. 5.5 - Why are throttling devices commonly used in...Ch. 5.5 - Would you expect the temperature of air to drop as...Ch. 5.5 - Prob. 60PCh. 5.5 - During a throttling process, the temperature of a...Ch. 5.5 - Refrigerant-134a is throttled from the saturated...Ch. 5.5 - A saturated liquidvapor mixture of water, called...Ch. 5.5 - Prob. 64PCh. 5.5 - A well-insulated valve is used to throttle steam...Ch. 5.5 - Refrigerant-134a enters the expansion valve of a...Ch. 5.5 - Prob. 68PCh. 5.5 - Consider a steady-flow heat exchanger involving...Ch. 5.5 - Prob. 70PCh. 5.5 - Prob. 71PCh. 5.5 - Prob. 72PCh. 5.5 - Prob. 73PCh. 5.5 - Prob. 74PCh. 5.5 - Prob. 76PCh. 5.5 - Steam is to be condensed on the shell side of a...Ch. 5.5 - Prob. 78PCh. 5.5 - Air (cp = 1.005 kJ/kgC) is to be preheated by hot...Ch. 5.5 - Prob. 80PCh. 5.5 - Refrigerant-134a at 1 MPa and 90C is to be cooled...Ch. 5.5 - Prob. 82PCh. 5.5 - An air-conditioning system involves the mixing of...Ch. 5.5 - The evaporator of a refrigeration cycle is...Ch. 5.5 - Steam is to be condensed in the condenser of a...Ch. 5.5 - Steam is to be condensed in the condenser of a...Ch. 5.5 - Two mass streams of the same ideal gas are mixed...Ch. 5.5 - Prob. 89PCh. 5.5 - A 110-volt electrical heater is used to warm 0.3...Ch. 5.5 - The fan on a personal computer draws 0.3 ft3/s of...Ch. 5.5 - Prob. 92PCh. 5.5 - 5–93 A scaled electronic box is to be cooled by...Ch. 5.5 - Prob. 94PCh. 5.5 - Prob. 95PCh. 5.5 - Prob. 96PCh. 5.5 - Prob. 97PCh. 5.5 - A computer cooled by a fan contains eight PCBs,...Ch. 5.5 - Prob. 99PCh. 5.5 - A long roll of 2-m-wide and 0.5-cm-thick 1-Mn...Ch. 5.5 - Prob. 101PCh. 5.5 - Prob. 102PCh. 5.5 - A house has an electric heating system that...Ch. 5.5 - Steam enters a long, horizontal pipe with an inlet...Ch. 5.5 - Refrigerant-134a enters the condenser of a...Ch. 5.5 - Prob. 106PCh. 5.5 - Water is heated in an insulated, constant-diameter...Ch. 5.5 - Prob. 108PCh. 5.5 - Air enters the duct of an air-conditioning system...Ch. 5.5 - A rigid, insulated tank that is initially...Ch. 5.5 - 5–113 A rigid, insulated tank that is initially...Ch. 5.5 - Prob. 114PCh. 5.5 - A 0.2-m3 rigid tank equipped with a pressure...Ch. 5.5 - Prob. 116PCh. 5.5 - Prob. 117PCh. 5.5 - Prob. 118PCh. 5.5 - Prob. 119PCh. 5.5 - An air-conditioning system is to be filled from a...Ch. 5.5 - Oxygen is supplied to a medical facility from ten...Ch. 5.5 - Prob. 122PCh. 5.5 - A 0.3-m3 rigid tank is filled with saturated...Ch. 5.5 - Prob. 124PCh. 5.5 - Prob. 125PCh. 5.5 - Prob. 126PCh. 5.5 - The air-release flap on a hot-air balloon is used...Ch. 5.5 - An insulated 0.15-m3 tank contains helium at 3 MPa...Ch. 5.5 - An insulated 40-ft3 rigid tank contains air at 50...Ch. 5.5 - A vertical pistoncylinder device initially...Ch. 5.5 - A vertical piston-cylinder device initially...Ch. 5.5 - Prob. 135RPCh. 5.5 - Prob. 136RPCh. 5.5 - Air at 4.18 kg/m3 enters a nozzle that has an...Ch. 5.5 - An air compressor compresses 15 L/s of air at 120...Ch. 5.5 - 5–139 Saturated refrigerant-134a vapor at 34°C is...Ch. 5.5 - A steam turbine operates with 1.6 MPa and 350C...Ch. 5.5 - Prob. 141RPCh. 5.5 - Prob. 142RPCh. 5.5 - Prob. 143RPCh. 5.5 - Steam enters a nozzle with a low velocity at 150C...Ch. 5.5 - Prob. 146RPCh. 5.5 - Prob. 147RPCh. 5.5 - Prob. 148RPCh. 5.5 - Prob. 149RPCh. 5.5 - Cold water enters a steam generator at 20C and...Ch. 5.5 - Prob. 151RPCh. 5.5 - An ideal gas expands in an adiabatic turbine from...Ch. 5.5 - Prob. 153RPCh. 5.5 - Prob. 154RPCh. 5.5 - Prob. 155RPCh. 5.5 - Prob. 156RPCh. 5.5 - Prob. 157RPCh. 5.5 - Prob. 158RPCh. 5.5 - Prob. 159RPCh. 5.5 - Prob. 160RPCh. 5.5 - Prob. 161RPCh. 5.5 - Prob. 162RPCh. 5.5 - Prob. 163RPCh. 5.5 - The ventilating fan of the bathroom of a building...Ch. 5.5 - Determine the rate of sensible heat loss from a...Ch. 5.5 - An air-conditioning system requires airflow at the...Ch. 5.5 - The maximum flow rate of standard shower heads is...Ch. 5.5 - An adiabatic air compressor is to be powered by a...Ch. 5.5 - Prob. 171RPCh. 5.5 - Prob. 172RPCh. 5.5 - Prob. 173RPCh. 5.5 - Prob. 174RPCh. 5.5 - Prob. 175RPCh. 5.5 - A tank with an internal volume of 1 m3 contains...Ch. 5.5 - A liquid R-134a bottle has an internal volume of...Ch. 5.5 - Prob. 179RPCh. 5.5 - Prob. 181RPCh. 5.5 - Prob. 182RPCh. 5.5 - Prob. 184RPCh. 5.5 - A pistoncylinder device initially contains 1.2 kg...Ch. 5.5 - In a single-flash geothermal power plant,...Ch. 5.5 - The turbocharger of an internal combustion engine...Ch. 5.5 - A building with an internal volume of 400 m3 is to...Ch. 5.5 - Prob. 189RPCh. 5.5 - Prob. 190RPCh. 5.5 - Prob. 191RPCh. 5.5 - Prob. 192FEPCh. 5.5 - Prob. 193FEPCh. 5.5 - An adiabatic heat exchanger is used to heat cold...Ch. 5.5 - A heat exchanger is used to heat cold water at 15C...Ch. 5.5 - An adiabatic heat exchanger is used to heat cold...Ch. 5.5 - In a shower, cold water at 10C flowing at a rate...Ch. 5.5 - Prob. 198FEPCh. 5.5 - Hot combustion gases (assumed to have the...Ch. 5.5 - Steam expands in a turbine from 4 MPa and 500C to...Ch. 5.5 - Steam is compressed by an adiabatic compressor...Ch. 5.5 - Refrigerant-134a is compressed by a compressor...Ch. 5.5 - Prob. 203FEPCh. 5.5 - Prob. 204FEPCh. 5.5 - Air at 27C and 5 atm is throttled by a valve to 1...Ch. 5.5 - Steam at 1 MPa and 300C is throttled adiabatically...Ch. 5.5 - Air is to be heated steadily by an 8-kW electric...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Qu. 17 Compute linear density values for [100] for silver (Ag). Express your answer in nm''. . Round off the answer to three significant figures. Qu. 18 Compute linear density value for [111] direction for silver (Ag). Express your answer in nm'. Round off the answer to three significant figures. Qu. 19 Compute planar density value for (100) plane for chromium (Cr). Express your answer in nm?. Round off the answer to two significant figures. Qu. 20 Compute planar density value for (110) plane for chromium (Cr). Express your answer in nm ≥ to four significant figures. show all work please in material engineeringarrow_forward3-142arrow_forwardI need solutionsarrow_forward
- 3-137arrow_forwardLarge wind turbines with a power capacity of 8 MW and blade span diameters of over 160 m areavailable for electric power generation. Consider a wind turbine with a blade span diameter of 120m installed at a site subjected to steady winds at 8.25 m/s. Taking the overall efficiency of thewind turbine to be 33 percent and the air density to be 1.25 kg/m3, determine the electric powergenerated by this wind turbine. Also, assuming steady winds of 8.25 m/s during a 24-h period,determine the amount of electric energy and the revenue generated per day for a unit price of$0.08/kWh for electricity.arrow_forwardThe basic barometer can be used to measure the height of a building. If the barometric readingsat the top and at the bottom of a building are 672 and 696 mmHg, respectively, determine theheight of the building. Take the densities of air and mercury to be 1.18 kg/m3 and 13,600 kg/m3,respectivelyarrow_forward
- A 7.25-hp (shaft) pump is used to raise water to an elevation of 17 m. If the mechanical efficiencyof the pump is 84 percent, determine the maximum volume flow rate of water.arrow_forwardConsider a double-fluid manometer attached to an air pipe shown below. If the specific gravity ofone fluid is 13.8, determine the specific gravity of the other fluid for the indicated absolutepressure of air. Take the atmospheric pressure to be 95 kPaarrow_forwardA race car enters the circular portion of a track that has a radius of 65 m. Disregard the 70 m in the picture. When the car enters the curve at point P, it is traveling with a speed of 120 km/h that is increasing at 5 m/s^2 . Three seconds later, determine the x and y components of velocity and acceleration of the car. I'm having trouble getting the correct y component of acceleration. all the other answers are correct. thank you!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=NyOYW07-L5g;License: Standard youtube license