
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5.12, Problem 36P
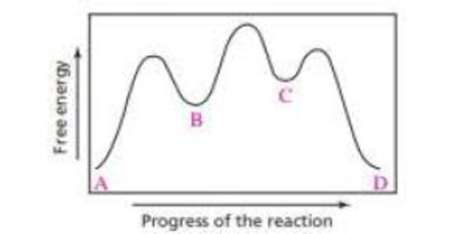
a. Which step in the reaction coordinate diagram shown here has the, greatest free energy of activation in the forward direction?
b. Is the first-formed intermediate more apt to revert to reactants or go on to form products?
c. Which step is the rate-determining step of the reaction?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the following energy diagrams is a reaction with one intermediate?
...
reaction coordinate
...
reaction coordinate
free energy,
kJ/mol
free energy,
kJ/mol
8. Draw a reaction coordinate diagram for ...
an endergonic reaction
***
***
Free energy
Free energy
Free energy
Progress of the reaction
a three-step reaction
Progress of the reaction
... a reaction that proceeds through two
intermediates
Progress of the reaction
Free energy
Progress of the reaction
a reaction that proceeds through one
transition state
***
Progress of the reaction
a two-step reaction, in which the
***
a two-step reaction, in which the first
second step is the rate-determining step step has the highest energy of activation
Free energy
Free energy
Progress of the reaction
If you use a catalyst to speed up the reaction, how will that affect the reaction coordinate?
Chapter 5 Solutions
EBK ORGANIC CHEMISTRY
Ch. 5.1 - What is the molecular formula for each of the...Ch. 5.1 - Prob. 4PCh. 5.1 - Determine the degree of unsaturation and then draw...Ch. 5.1 - Prob. 6PCh. 5.2 - What is each compounds systematic name?Ch. 5.2 - Prob. 8PCh. 5.2 - Draw the structure for each of the following: a....Ch. 5.3 - How many carbons are in the planar double-bond...Ch. 5.3 - Prob. 12PCh. 5.5 - Prob. 13P
Ch. 5.5 - Prob. 14PCh. 5.5 - Prob. 16PCh. 5.5 - Prob. 17PCh. 5.6 - a. Which of the monosubstituted cyclohexanes in...Ch. 5.6 - a. Calculate the percentage of isopropylcylohexane...Ch. 5.6 - a. for which reaction in each set will S be more...Ch. 5.6 - a. For a reaction with H = 12 kcal/ mol and S =...Ch. 5.8 - Prob. 23PCh. 5.9 - Prob. 24PCh. 5.9 - How many different alkenes can be hydrogenated to...Ch. 5.9 - The same alkane is obtained from the catalytic...Ch. 5.9 - Prob. 27PCh. 5.9 - Rank the following compounds from most stable to...Ch. 5.10 - Prob. 29PCh. 5.10 - Prob. 30PCh. 5.11 - The rate constant for a reaction can be increased...Ch. 5.11 - Prob. 33PCh. 5.11 - a. Which reaction has a greater equilibrium...Ch. 5.12 - Draw a reaction coordinate diagram for a two-step...Ch. 5.12 - a. Which step in the reaction coordinate diagram...Ch. 5.12 - Draw a reaction coordinate diagram for the...Ch. 5.13 - Prob. 38PCh. 5 - What is each compounds systematic name?Ch. 5 - Prob. 40PCh. 5 - Draw the structure of a hydrocarbon that has six...Ch. 5 - Draw the condensed structure for each of the...Ch. 5 - Prob. 43PCh. 5 - Prob. 44PCh. 5 - Prob. 45PCh. 5 - Name the following:Ch. 5 - Prob. 47PCh. 5 - Prob. 48PCh. 5 - Prob. 49PCh. 5 - In a reaction in which reactant A is in...Ch. 5 - Which bond is stronger? Briefly explain why.Ch. 5 - Prob. 52PCh. 5 - Prob. 53PCh. 5 - By following the curved red arrows, draw the...Ch. 5 - Prob. 55PCh. 5 - Prob. 56PCh. 5 - Draw structures for the following: a....Ch. 5 - Prob. 58PCh. 5 - a. Which of the following reactions has the larger...Ch. 5 - Prob. 60PCh. 5 - a. What is the equilibrium constant for a reaction...Ch. 5 - Prob. 62PCh. 5 - Prob. 63PCh. 5 - Given that the free energy of the twist-boat...Ch. 5 - Prob. 65PCh. 5 - Prob. 1PCh. 5 - Prob. 2PCh. 5 - Prob. 3PCh. 5 - Prob. 4PCh. 5 - Prob. 5PCh. 5 - Prob. 6PCh. 5 - Draw curved arrows to show the movement of the...Ch. 5 - Prob. 8PCh. 5 - Prob. 9PCh. 5 - Prob. 10P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The rate determining step of a reaction is the slowest step and thus, has the lowest energy barrier first step on a staircase fastest step and thus, has the lowest energy barrier slowest step and thus, has the highest energy barrier fastest step and thus, has the highest energy barrierarrow_forwardOutlino how and why increasing tomporature, concontration, and a catalyst can speed up the rato of a reaction.arrow_forwardHello. Can you explain what happens to rate of reaction as the temperature increases.arrow_forward
- Please don't provide handwritten solution ....arrow_forwardEl E2 Example Reaction Equation Rate Law Energy Diagram Why El or E2? Explainarrow_forwardWhat is the effect on the rate constant and reaction rate when reaction temperature is increased? The rate constant will be greater, and the reaction rate will be higher. The rate constant will be smaller, but the reaction rate will be higher. The rate constant will be smaller, and the reaction rate will be lower.arrow_forward
- Rank the relative rates of the three steps for the multistep mechanism. Step1, step 2, step 3 Which step have slowest and fastest relative speedarrow_forward17. The rate-determining step for a chemical reaction that proceeds by multiple mechanistic steps, is the step: a) with the highest energy transition state. b) with the largest activation energy. c) with a transition state that looks like the starting material for the mechanistic step. d) with a transition state that looks like the product for the mechanistic step.arrow_forward7) Relates the reaction rate to the concentrations of reactants. 9. General Rate Reaction C Raje Law b. Rate of of Reaction d Order of the Reactionarrow_forward
- Compare all four energy diagrams. Which one exhibits the largest Ea and which processes will have a value of Keq that is greater than 1?arrow_forward6. Draw a detailed free energy diagram for the following reaction. Include and label the overall reactants, the overall products, the intermediate(s), and the axis, activation energies, transition state(s) and overall Gibbs free energy. HOC(CH₂) E1 Draw the Intermediate Draw the Productarrow_forwardComplete each sentence by matching with the correct statement.Increasing the concentration of a reactant: a. Increases the frequency and energy of molecular collisions which speeds up the rate of the reactionb. Reduces the energy and frequency of the collisions thus slowing down the rate of the reactionc. Increases the frequency of molecular collisions thus speeding up the rate of the reactiond. Increases the activation thus speeding up the rate of the reactione. Lowers the activation energy and speeds up the reactionf. Gives the molecules more energyAdding a catalyst to a reaction:a. Increases the frequency and energy of molecular collisions which speeds up the rate of the reactionb. Reduces the energy and frequency of the collisions thus slowing down the rate of the reactionc. Increases the frequency of molecular collisions thus speeding up the rate of the reactiond. Increases the activation thus speeding up the rate of the reactione. Lowers the activation energy and speeds up the…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY