
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 7P
Draw curved arrows to show the movement of the electrons that result in formation of the given product(s).
a. 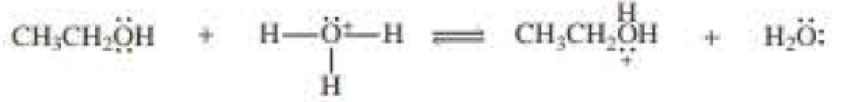
b. 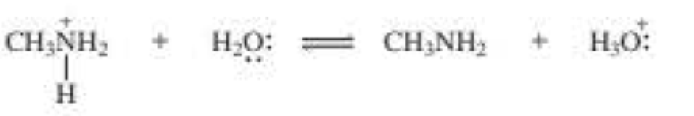
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What product results from the SN2 reaction between (R)-2-chloropentane and hydroxide?
A.
(R)-2-pentanol
B.
(S)-2-pentanol
C.
Both A and B
D.
1-pentanol
44
How many double bonds would be in one of the resonance structure of aromatic napthalene?
a.
c.
b. 6
d. 12
.is opposite of
A. Addition reaction.
B. Substation reaction
C. Elimination reaction.
Method is preparing 5
compounds with double and triple
.bonds is
A. Elimination reaction.
B. Substation reaction
C. Addition reaction.
which involve by
migration of atom or group from
.anther
A. Substation reaction.
B. Elimination reaction.
C. Rearrangement reaction.
D. Addition reaction.
Chapter 5 Solutions
EBK ORGANIC CHEMISTRY
Ch. 5.1 - What is the molecular formula for each of the...Ch. 5.1 - Prob. 4PCh. 5.1 - Determine the degree of unsaturation and then draw...Ch. 5.1 - Prob. 6PCh. 5.2 - What is each compounds systematic name?Ch. 5.2 - Prob. 8PCh. 5.2 - Draw the structure for each of the following: a....Ch. 5.3 - How many carbons are in the planar double-bond...Ch. 5.3 - Prob. 12PCh. 5.5 - Prob. 13P
Ch. 5.5 - Prob. 14PCh. 5.5 - Prob. 16PCh. 5.5 - Prob. 17PCh. 5.6 - a. Which of the monosubstituted cyclohexanes in...Ch. 5.6 - a. Calculate the percentage of isopropylcylohexane...Ch. 5.6 - a. for which reaction in each set will S be more...Ch. 5.6 - a. For a reaction with H = 12 kcal/ mol and S =...Ch. 5.8 - Prob. 23PCh. 5.9 - Prob. 24PCh. 5.9 - How many different alkenes can be hydrogenated to...Ch. 5.9 - The same alkane is obtained from the catalytic...Ch. 5.9 - Prob. 27PCh. 5.9 - Rank the following compounds from most stable to...Ch. 5.10 - Prob. 29PCh. 5.10 - Prob. 30PCh. 5.11 - The rate constant for a reaction can be increased...Ch. 5.11 - Prob. 33PCh. 5.11 - a. Which reaction has a greater equilibrium...Ch. 5.12 - Draw a reaction coordinate diagram for a two-step...Ch. 5.12 - a. Which step in the reaction coordinate diagram...Ch. 5.12 - Draw a reaction coordinate diagram for the...Ch. 5.13 - Prob. 38PCh. 5 - What is each compounds systematic name?Ch. 5 - Prob. 40PCh. 5 - Draw the structure of a hydrocarbon that has six...Ch. 5 - Draw the condensed structure for each of the...Ch. 5 - Prob. 43PCh. 5 - Prob. 44PCh. 5 - Prob. 45PCh. 5 - Name the following:Ch. 5 - Prob. 47PCh. 5 - Prob. 48PCh. 5 - Prob. 49PCh. 5 - In a reaction in which reactant A is in...Ch. 5 - Which bond is stronger? Briefly explain why.Ch. 5 - Prob. 52PCh. 5 - Prob. 53PCh. 5 - By following the curved red arrows, draw the...Ch. 5 - Prob. 55PCh. 5 - Prob. 56PCh. 5 - Draw structures for the following: a....Ch. 5 - Prob. 58PCh. 5 - a. Which of the following reactions has the larger...Ch. 5 - Prob. 60PCh. 5 - a. What is the equilibrium constant for a reaction...Ch. 5 - Prob. 62PCh. 5 - Prob. 63PCh. 5 - Given that the free energy of the twist-boat...Ch. 5 - Prob. 65PCh. 5 - Prob. 1PCh. 5 - Prob. 2PCh. 5 - Prob. 3PCh. 5 - Prob. 4PCh. 5 - Prob. 5PCh. 5 - Prob. 6PCh. 5 - Draw curved arrows to show the movement of the...Ch. 5 - Prob. 8PCh. 5 - Prob. 9PCh. 5 - Prob. 10P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the Functional Group and Reagent to Identity the Type of Reaction Draw the product of each reaction.arrow_forwardConsider the structure of cyclobutyne, if it undergoes hydration, which of the following final product is formed? a. Cyclobutane b. Cyclobutanol c. Cyclobutenol d. Cyclobutanonearrow_forwardI need question 1arrow_forward
- Cyclopentanone is treated with chlorine (Cl2) in the presence of acid (H*). What product is formed? A. Chlorocyclopentane B. 1-chloro-1-hydroxycyclopentane C. 2-chlorocyclopentanone D. 3-chlorocyclopentanonearrow_forwardDraw the structure of the ff. aldehydes and ketones. a. 3-chlorobutanal 6. 8,8-dibromo -4-cthylcyclo hexanone C. 2,4-dimetnylpontanone • Draw the structure of the ff. compounds. a. oyclobutanecarboxylic acid b. 3,8-dimcthylpontancdioic acid C. 4-aminopcntanoic acid d. 2-mothylcycloheranecarooxylic acid m- chlorobėnzoic acidarrow_forwardGive the IUPAC or common name for attached compoundarrow_forward
- Which of the following reacts fastest in nucleophilic addition reaction? a. Acetic acid b. Ethanol c. Formaldehyde d. Benzaldehyde e. Acetone How can a water insoluble acid be converted into a water soluble compound? a. By reacting it with an alcohol. b. By reacting it with hydrochloric acid. c. By reacting it with nitric acid. d. None of the mentioned method. e. By reacting it with sodium bicarbonatearrow_forwardDraw the structure corresponding to each IUPAC namearrow_forwardBe sure to answer all parts. Draw the structure corresponding to each IUPAC name. a. propylcyclopentane draw structure... b. 1,1,2-trimethylcyclopropane draw structure...arrow_forward
- Consider the molecule: rate the priority functional groups from highest to lowest A. Alkyl chain B. Ketone C. Carbonyl D. Anhydridearrow_forwardBe sure to answer all parts. Draw the structure corresponding to each IUPAC name. a. 6-butyl-3-methyldecane CH₂ edit structure ... b. 4,4,5,5-tetramethylnonane X H₂C₂ CH₂ edit structure ...arrow_forwardWhat is the IUPAC name? CH, a. (S)-4-methylpent-1-ynal b. (R)-2-methylpent-4-yank c. (S)-2-methylpent-4-yank d. (R)-4-methylpent-1-ynalarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY