
Concept explainers
Interpretation:
The suffix that is used to name the given compound has to be identified.
Concept Introduction:
All the molecules have their unique names. These names must be known in order to communicate. But remembering all the chemical names is impossible as there are many numbers of molecules. To avoid this, the
| Stereoisomerism | Substituents | Parent | Unsaturation | Functional Group |
Stereoisomerism indicate if the considered molecule has any stereocenters are present (R,S) and if double bond is present are cis/trans.
The groups that are connected to the main carbon chain is known as substituents.
The longest carbon chain is known as the parent.
Unsaturation indicates that if any triple or double bonds are present in the molecule.
Functional group is the one after which the considered compound is being named.
| Functional Group | Class of compound | Suffix |
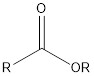 | Ester | -oate |
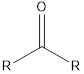 | -one | |
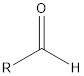 | -al | |
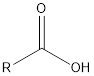 | -oic acid | |
 | Alcohol | -ol |
 | -amine |
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry As a Second Language: First Semester Topics
- Pls help asaparrow_forwardPls help asaparrow_forwardPredict the major products of this reaction: ་ ་ + H NaOH ? Δ excess Note that the second reactant is used in excess, that is, there is much more of the second reactant than the first. If there won't be any products, just check the box under the drawing area instead.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





