
Thermodynamics: An Engineering Approach
9th Edition
ISBN: 9781259822674
Author: Yunus A. Cengel Dr., Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.5, Problem 38P
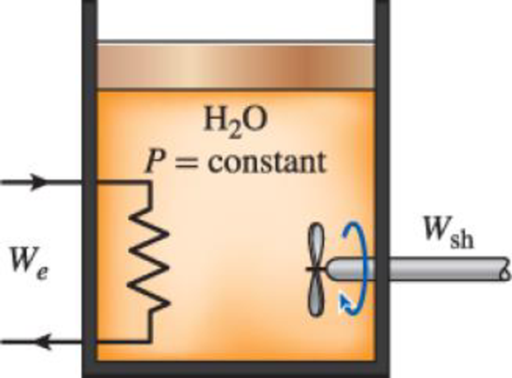
An insulated piston–cylinder device contains 5 L of saturated liquid water at a constant pressure of 175 kPa. Water is stirred by a paddle wheel while a current of 8 A flows for 45 min through a resistor placed in the water. If one-half of the liquid is evaporated during this constant-pressure process and the paddle-wheel work amounts to 400 kJ, determine the voltage of the source. Also, show the process on a P-V diagram with respect to saturation lines.
FIGURE P4–38
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A virtual experiment is designed to determine the effect of friction on the timing and speed
of packages being delivered to a conveyor belt and the normal force applied to the tube.
A package is held and then let go at the edge of a circular shaped tube of radius R = 5m.
The particle at the bottom will transfer to the conveyor belt, as shown below.
Run the simulations for μ = 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6 and determine the time and speed at
which the package is delivered to the conveyor belt. In addition, determine the maximum
normal force and its location along the path as measured by angle 0.
Submit in hardcopy form:
(0) Free Body Diagram, equations underneath, derivations
(a) Your MATLAB mfile
(b) A table listing the values in 5 columns:
μ, T (time of transfer), V (speed of transfer), 0 (angle of max N), Nmax (max N)
(c) Based on your results, explain in one sentence what you think will happen to the
package if the friction is increased even further, e.g. μ = 0.8.
NOTE: The ODE is…
Patm = 1 bar
Piston
m = 50 kg
5 g of Air
T₁ = 600 K
P₁ = 3 bar
Stops
A 9.75 x 10-3 m²
FIGURE P3.88
Assume a Space Launch System (Figure 1(a)) that is approximated as a cantilever undamped single degree of freedom (SDOF) system with a mass at its free end (Figure 1(b)). The cantilever is assumed to be massless. Assume a wind load that is approximated with a concentrated harmonic forcing function p(t) = posin(ωt) acting on the mass. The known properties of the SDOF and the applied forcing function are given below. • Mass of SDOF: m =120 kip/g • Acceleration of gravity: g = 386 in/sec2 • Bending sectional stiffness of SDOF: EI = 1015 lbf×in2 • Height of SDOF: h = 2000 inches • Amplitude of forcing function: po = 6 kip • Forcing frequency: f = 8 H
Chapter 4 Solutions
Thermodynamics: An Engineering Approach
Ch. 4.5 - Is the boundary work associated with...Ch. 4.5 - On a P-V diagram, what does the area under the...Ch. 4.5 - An ideal gas at a given state expands to a fixed...Ch. 4.5 - Calculate the total work, in kJ, for process 13...Ch. 4.5 - Calculate the total work, in Btu, produced by the...Ch. 4.5 - Nitrogen at an initial state of 300 K, 150 kPa,...Ch. 4.5 - The volume of 1 kg of helium in a pistoncylinder...Ch. 4.5 - A pistoncylinder device with a set of stops...Ch. 4.5 - A mass of 5 kg of saturated water vapor at 150 kPa...Ch. 4.5 - A frictionless pistoncylinder device contains 16...
Ch. 4.5 - 1 m3 of saturated liquid water at 200C is expanded...Ch. 4.5 - Argon is compressed in a polytropic process with n...Ch. 4.5 - A gas is compressed from an initial volume of 0.42...Ch. 4.5 - A mass of 1.5 kg of air at 120 kPa and 24C is...Ch. 4.5 - During some actual expansion and compression...Ch. 4.5 - A frictionless pistoncylinder device contains 5 kg...Ch. 4.5 - During an expansion process, the pressure of a gas...Ch. 4.5 - A pistoncylinder device initially contains 0.4 kg...Ch. 4.5 - A pistoncylinder device contains 0.15 kg of air...Ch. 4.5 - Determine the boundary work done by a gas during...Ch. 4.5 - 1 kg of water that is initially at 90C with a...Ch. 4.5 - An ideal gas undergoes two processes in a...Ch. 4.5 - A pistoncylinder device contains 50 kg of water at...Ch. 4.5 - Prob. 26PCh. 4.5 - A closed system like that shown in Fig. P427E is...Ch. 4.5 - A rigid container equipped with a stirring device...Ch. 4.5 - Complete each line of the following table on the...Ch. 4.5 - A substance is contained in a well-insulated rigid...Ch. 4.5 - A 0.5-m3rigid tank contains refrigerant-134a...Ch. 4.5 - A 20-ft3 rigid tank initially contains saturated...Ch. 4.5 - A rigid 10-L vessel initially contains a mixture...Ch. 4.5 - A rigid 1-ft3 vessel contains R-134a originally at...Ch. 4.5 - A pistoncylinder device contains 5 kg of...Ch. 4.5 - A pistoncylinder device contains 0.5 lbm of water...Ch. 4.5 - 2 kg of saturated liquid water at 150C is heated...Ch. 4.5 - An insulated pistoncylinder device contains 5 L of...Ch. 4.5 - A 40-L electrical radiator containing heating oil...Ch. 4.5 - Steam at 75 kPa and 8 percent quality is contained...Ch. 4.5 - A pistoncylinder device initially contains 0.6 m3...Ch. 4.5 - An insulated tank is divided into two parts by a...Ch. 4.5 - Two tanks (Tank A and Tank B) are separated by a...Ch. 4.5 - Is the energy required to heat air from 295 to 305...Ch. 4.5 - A fixed mass of an ideal gas is heated from 50 to...Ch. 4.5 - A fixed mass of an ideal gas is heated from 50 to...Ch. 4.5 - A fixed mass of an ideal gas is heated from 50 to...Ch. 4.5 - Is the relation u = mcv,avgT restricted to...Ch. 4.5 - Is the relation h = mcp,avgT restricted to...Ch. 4.5 - What is the change in the internal energy, in...Ch. 4.5 - Neon is compressed from 100 kPa and 20C to 500 kPa...Ch. 4.5 - What is the change in the enthalpy, in kJ/kg, of...Ch. 4.5 - A mass of 10 g of nitrogen is contained in the...Ch. 4.5 - Determine the internal energy change u of...Ch. 4.5 - Determine the enthalpy change h of oxygen, in...Ch. 4.5 - Is it possible to compress an ideal gas...Ch. 4.5 - Nitrogen in a rigid vessel is cooled by rejecting...Ch. 4.5 - Nitrogen at 100 psia and 300F in a rigid container...Ch. 4.5 - A pistoncylinder device containing carbon-dioxide...Ch. 4.5 - A 3-m3 rigid tank contains hydrogen at 250 kPa and...Ch. 4.5 - 1 kg of oxygen is heated from 20 to 120C....Ch. 4.5 - A 10-ft3 tank contains oxygen initially at 14.7...Ch. 4.5 - A 4-m 5-m 7-m room is heated by the radiator of...Ch. 4.5 - An insulated rigid tank is divided into two equal...Ch. 4.5 - An ideal gas contained in a pistoncylinder device...Ch. 4.5 - A 4-m 5-m 6-m room is to be heated by a...Ch. 4.5 - An insulated pistoncylinder device initially...Ch. 4.5 - Argon is compressed in a polytropic process with n...Ch. 4.5 - An insulated pistoncylinder device contains 100 L...Ch. 4.5 - Air is contained in a variable-load pistoncylinder...Ch. 4.5 - A mass of 15 kg of air in a pistoncylinder device...Ch. 4.5 - Prob. 73PCh. 4.5 - A pistoncylinder device contains 2.2 kg of...Ch. 4.5 - A pistoncylinder device contains 4 kg of argon at...Ch. 4.5 - A spring-loaded pistoncylinder device contains 5...Ch. 4.5 - Prob. 78PCh. 4.5 - Prob. 79PCh. 4.5 - A 1-kg block of iron is heated from 25 to 75C....Ch. 4.5 - The state of liquid water is changed from 50 psia...Ch. 4.5 - During a picnic on a hot summer day, all the cold...Ch. 4.5 - An ordinary egg can be approximated as a...Ch. 4.5 - Consider a 1000-W iron whose base plate is made of...Ch. 4.5 - Stainless steel ball bearings ( = 8085 kg/m3 and...Ch. 4.5 - In a production facility, 1.6-in-thick 2-ft 2-ft...Ch. 4.5 - Long cylindrical steel rods ( = 7833 kg/m3 and cp...Ch. 4.5 - An electronic device dissipating 25 W has a mass...Ch. 4.5 - Prob. 90PCh. 4.5 - Prob. 91PCh. 4.5 - Is the metabolizable energy content of a food the...Ch. 4.5 - Is the number of prospective occupants an...Ch. 4.5 - Prob. 94PCh. 4.5 - Prob. 95PCh. 4.5 - Prob. 96PCh. 4.5 - Consider two identical 80-kg men who are eating...Ch. 4.5 - A 68-kg woman is planning to bicycle for an hour....Ch. 4.5 - A 90-kg man gives in to temptation and eats an...Ch. 4.5 - A 60-kg man used to have an apple every day after...Ch. 4.5 - Consider a man who has 20 kg of body fat when he...Ch. 4.5 - Consider two identical 50-kg women, Candy and...Ch. 4.5 - Prob. 103PCh. 4.5 - Prob. 104PCh. 4.5 - Prob. 105PCh. 4.5 - Prob. 106PCh. 4.5 - Prob. 107PCh. 4.5 - Prob. 108PCh. 4.5 - Prob. 109RPCh. 4.5 - Prob. 110RPCh. 4.5 - Prob. 111RPCh. 4.5 - Prob. 112RPCh. 4.5 - Prob. 113RPCh. 4.5 - Consider a pistoncylinder device that contains 0.5...Ch. 4.5 - Prob. 115RPCh. 4.5 - Air in the amount of 2 lbm is contained in a...Ch. 4.5 - Air is expanded in a polytropic process with n =...Ch. 4.5 - Nitrogen at 100 kPa and 25C in a rigid vessel is...Ch. 4.5 - Prob. 119RPCh. 4.5 - A mass of 3 kg of saturated liquidvapor mixture of...Ch. 4.5 - A mass of 12 kg of saturated refrigerant-134a...Ch. 4.5 - Prob. 122RPCh. 4.5 - A pistoncylinder device contains helium gas...Ch. 4.5 - Prob. 124RPCh. 4.5 - Prob. 125RPCh. 4.5 - Prob. 126RPCh. 4.5 - Prob. 127RPCh. 4.5 - Water is boiled at sea level in a coffeemaker...Ch. 4.5 - The energy content of a certain food is to be...Ch. 4.5 - Prob. 130RPCh. 4.5 - An insulated pistoncylinder device initially...Ch. 4.5 - An insulated rigid tank initially contains 1.4 kg...Ch. 4.5 - In order to cool 1 ton of water at 20C in an...Ch. 4.5 - A 0.3-L glass of water at 20C is to be cooled with...Ch. 4.5 - A well-insulated 3-m 4m 6-m room initially at 7C...Ch. 4.5 - Prob. 137RPCh. 4.5 - Prob. 138RPCh. 4.5 - Prob. 140RPCh. 4.5 - A pistoncylinder device initially contains 0.35 kg...Ch. 4.5 - Two 10-ft3 adiabatic tanks are connected by a...Ch. 4.5 - Prob. 143RPCh. 4.5 - Prob. 144RPCh. 4.5 - A 3-m3 rigid tank contains nitrogen gas at 500 kPa...Ch. 4.5 - A 0.5-m3 rigid tank contains nitrogen gas at 600...Ch. 4.5 - A well-sealed room contains 60 kg of air at 200...Ch. 4.5 - A room contains 75 kg of air at 100 kPa and 15C....Ch. 4.5 - Prob. 149FEPCh. 4.5 - A pistoncylinder device contains 5 kg of air at...Ch. 4.5 - Prob. 151FEPCh. 4.5 - A 2-kW electric resistance heater submerged in 5...Ch. 4.5 - Prob. 153FEPCh. 4.5 - 1.5 kg of liquid water initially at 12C is to be...Ch. 4.5 - Prob. 155FEPCh. 4.5 - An ordinary egg with a mass of 0.1 kg and a...Ch. 4.5 - Prob. 157FEPCh. 4.5 - A 6-pack of canned drinks is to be cooled from 18C...Ch. 4.5 - Prob. 159FEPCh. 4.5 - An ideal gas has a gas constant R = 0.3 kJ/kgK and...Ch. 4.5 - A pistoncylinder device contains an ideal gas. The...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Assume a Space Launch System (Figure 1(a)) that is approximated as a cantilever undamped single degree of freedom (SDOF) system with a mass at its free end (Figure 1(b)). The cantilever is assumed to be massless. Assume a wind load that is approximated with a concentrated harmonic forcing function p(t) = posin(ωt) acting on the mass. The known properties of the SDOF and the applied forcing function are given below. • Mass of SDOF: m =120 kip/g • Acceleration of gravity: g = 386 in/sec2 • Bending sectional stiffness of SDOF: EI = 1015 lbf×in2 • Height of SDOF: h = 2000 inches • Amplitude of forcing function: po = 6 kip • Forcing frequency: f = 8 Hz Figure 1: Single-degree-of-freedom system in Problem 1. Please compute the following considering the steady-state response of the SDOF system. Do not consider the transient response unless it is explicitly stated in the question. (a) The natural circular frequency and the natural period of the SDOF. (10 points) (b) The maximum displacement of…arrow_forwardAssume a Space Launch System (Figure 1(a)) that is approximated as a cantilever undamped single degree of freedom (SDOF) system with a mass at its free end (Figure 1(b)). The cantilever is assumed to be massless. Assume a wind load that is approximated with a concentrated harmonic forcing function p(t) = posin(ωt) acting on the mass. The known properties of the SDOF and the applied forcing function are given below. • Mass of SDOF: m =120 kip/g • Acceleration of gravity: g = 386 in/sec2 • Bending sectional stiffness of SDOF: EI = 1015 lbf×in2 • Height of SDOF: h = 2000 inches • Amplitude of forcing function: po = 6 kip • Forcing frequency: f = 8 Hz Figure 1: Single-degree-of-freedom system in Problem 1. Please compute the following considering the steady-state response of the SDOF system. Do not consider the transient response unless it is explicitly stated in the question. (a) The natural circular frequency and the natural period of the SDOF. (10 points) (b) The maximum displacement of…arrow_forwardPlease solve 13 * √(2675.16)² + (63.72 + 2255,03)² = 175x106 can you explain the process for getting d seperate thank youarrow_forward
- If the 300-kg drum has a center of mass at point G, determine the horizontal and vertical components of force acting at pin A and the reactions on the smooth pads C and D. The grip at B on member DAB resists both horizontal and vertical components of force at the rim of the drum. P 60 mm; 60 mm: 600 mm A E 30° B C 390 mm 100 mm D Garrow_forwardThe design of the gear-and-shaft system shown requires that steel shafts of the same diameter be used for both AB and CD. It is further required that the angle D through which end D of shaft CD rotates not exceed 1.5°. Knowing that G = 77.2 GPa, determine the required diameter of the shafts. 40 mm 400 mm 100 mm 600 mm T-1000 N-m Darrow_forwardAssume a Space Launch System (Figure 1(a)) that is approximated as a cantilever undamped single degree of freedom (SDOF) system with a mass at its free end (Figure 1(b)). The cantilever is assumed to be massless. Assume a wind load that is approximated with a concentrated harmonic forcing function p(t) = posin(ωt) acting on the mass. The known properties of the SDOF and the applied forcing function are given below. • Mass of SDOF: m =120 kip/g • Acceleration of gravity: g = 386 in/sec2 • Bending sectional stiffness of SDOF: EI = 1015 lbf×in2 • Height of SDOF: h = 2000 inches • Amplitude of forcing function: po = 6 kip • Forcing frequency: f = 8 Hzarrow_forward
- 13.44 The end of a cylindrical liquid cryogenic propellant tank in free space is to be protected from external (solar) radiation by placing a thin metallic shield in front of the tank. Assume the view factor Fts between the tank and the shield is unity; all surfaces are diffuse and gray, and the surroundings are at 0 K. Tank T₁ Shield, T T₁ = 100 K E1 Solar irradiation Gs ε₁ = ε₂ = 0.05 ε₁ = 0.10 Gs = 1250 W/m² E2 Find the temperature of the shield T, and the heat flux (W/m²) to the end of the tank.arrow_forwardquestion 664 thank youarrow_forward13.38 Consider the attic of a home located in a hot climate. The floor of the attic is characterized by a width of L₁ = 8 m while the roof makes an angle of 0 = 30° from the horizontal direction, as shown in the schematic. The homeowner wishes to reduce the heat load to the home by adhering bright aluminum foil (ε = 0.07) onto the surfaces of the attic space. Prior to installation of the foil, the surfaces are of emissivity & = 0.90. Attic A2, 82, T2 0 = 30° A1, E1, T₁ 土 L₁ = 8 m (a) Consider installation on the bottom of the attic roof only. Determine the ratio of the radiation heat transfer after to before the installation of the foil. (b) Determine the ratio of the radiation heat transfer after to before installation if the foil is installed only on the top of the attic floor. (c) Determine the ratio of the radiation heat transfer if the foil is installed on both the roof bottom and the floor top.arrow_forward
- 13.1 Determine F2 and F2 for the following configura- tions using the reciprocity theorem and other basic shape factor relations. Do not use tables or charts. (a) Small sphere of area A, under a concentric hemi- sphere of area A₂ = 3A₁ A₂ A1 (a) (b) Long duct. Also, what is F₁₂? A₂ Αν (b) (c) Long inclined plates (point B is directly above the center of A₁) B 100 mm A₂ - 220 mm (c) (d) Long cylinder lying on infinite plane + A₁ Az (d) (e) Hemisphere-disk arrangement -A₂, hemisphere, diameter D A₂ A₁, disk, diameter D/2 (e) (f) Long, open channel 1 m AA₂ 2 m (f) (g) Long cylinders with A₁ = 4A₁. Also, what is F₁₂? -D₁ A1 -A₂ -D2 (e) (h) Long, square rod in a long cylinder. Also, what is F22? w=D/5 18 A₁ -A2 (h) -Darrow_forward13.9 Determine the shape factor, F12, for the rectangles shown. 6 m 1 3 m 6 m 1 m 2 6 m 1 0.5 m 2 1 m (a) Perpendicular rectangles without a common edge. -1 m. (b) Parallel rectangles of unequal areas.arrow_forwardI keep getting the wrong answer i have gotten 6519.87 and 319.71arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Thermodynamics - Chapter 3 - Pure substances; Author: Engineering Deciphered;https://www.youtube.com/watch?v=bTMQtj13yu8;License: Standard YouTube License, CC-BY