
Concept explainers
A well-insulated 3-m × 4–m × 6-m room initially at 7°C is heated by the radiator of a steam heating system. The radiator has a volume of 15 L and is filled with superheated vapor at 200 kPa and 200°C. At this moment both the inlet and the exit valves to the radiator are closed. A 120-W fan is used to distribute the air in the room. The pressure of the steam is observed to drop to 100 kPa after 45 min as a result of heat transfer to the room. Assuming constant specific heats for air at room temperature, determine the average temperature of air in 45 min. Assume the air pressure in the room remains constant at 100 kPa.
FIGURE P4–136
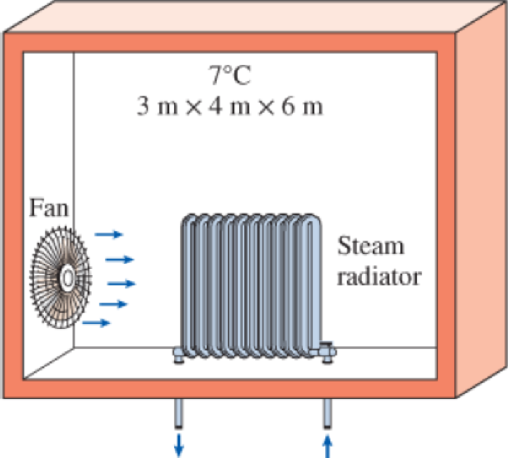
The average temperature of air in 45 min.
Answer to Problem 136RP
The average temperature of air in 45 min is
Explanation of Solution
Show the free body diagram of the well-insulated room.
Write the expression for the energy balance equation.
Here, the total energy entering the system is
Substitute
Here, the mass is
From the Table (A-4 through A-6), obtain the value of specific volume, specific internal energy at the initial and the final states.
At initial pressure and temperature of
At final pressure of
Write the expression for final quality at the final state.
Here, the specific volume of saturated liquid is
Write the expression for final specific internal energy of a well-insulated room.
Here, the specific internal energy of saturated liquid is
Write the expression for total mass of a well-insulated room.
Here, the initial specific volume is
Write expression for the volume of the well-insulated room.
Write the expression of mass of air in a well-insulated room.
Here, the initial pressure is
Write the expression for amount of fan work done in 45 min.
Conclusion:
Here, the specific volume
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
For well-insulated room the energy balance equation.\
Substitute
Express the change in internal energy and boundary work into the constant pressure expansion and compression in Equation (IX).
Here, the rate of heat transfer entering is
Substitute
Therefore, the well-insulated room temperature rises from
Thus, the average temperature of air in 45 min is
Want to see more full solutions like this?
Chapter 4 Solutions
Thermodynamics: An Engineering Approach
- The members of a truss are connected to the gusset plate as shown in (Figure 1). The forces are concurrent at point O. Take = 90° and T₁ = 7.5 kN. Part A Determine the magnitude of F for equilibrium. Express your answer to three significant figures and include the appropriate units. F= 7.03 Submit ? kN Previous Answers Request Answer × Incorrect; Try Again; 21 attempts remaining ▾ Part B Determine the magnitude of T2 for equilibrium. Express your answer to three significant figures and include the appropriate units. Figure T₂ = 7.03 C T2 |? KN Submit Previous Answers Request Answer × Incorrect; Try Again; 23 attempts remaining Provide Feedbackarrow_forwardConsider the following acid-base reaction: Fe3+(aq) +3H2O -Fe(OH)3 (s) + 3H* ← A. Using thermodynamics, calculate the equilibrium constant K at 25°C (The AG° of formation of Fe(OH)3(s) is -699 kJ/mol). B. Using the value of K you calculated in part a, if a solution contains 10-4 M Fe3+ and has a pH of 7.5, will Fe(OH)3(s) precipitate? Show all calculations necessary to justify your answer. Note that the reaction as written is for precipitation, not dissolution like Ksp-arrow_forwardA vertical force of F = 3.4 kN is applied to the hook at A as shown in. Set d = 1 m. Part A 3 m 3m 0.75 m 1.5 m. Determine the tension in cable AB for equilibrium. Express your answer to three significant figures and include the appropriate units. FAB= Value Submit Request Answer Part B Units ? Determine the tension in cable AC for equilibrium. Express your answer to three significant figures and include the appropriate units. FAC = Value Submit Request Answer Part C ? Units Determine the tension in cable AD for equilibrium. Express your answer to three significant figures and include the appropriate units.arrow_forward
- Consider the heat engine operating at steady state between the two thermal reservoirs shown at the right while producing a net power output of 700 kW. If 1000 kW of heat (Q̇H) is transferred to the heat engine from a thermal reservoir at a temperature of TH = 900 K, and heat is rejected to a thermal reservoir at a temperature of TL = 300 K, is this heat engine possible? Can you answer this question for me and show all of the workarrow_forward1.12 A disk of constant radius r is attached to a telescoping rod that is extending at a constant rate as shown in Fig. P1.12. Both the disk and the rod are rotating at a constant rate. Find the inertial velocity and acceleration of point P at the rim of the disk. ท2 L 0 SS P α e 0 O' êL Fig. P1.12 Rotating disk attached to telescoping rod. 60 LLarrow_forwardTwo different options A and B with brake pads for disc brakes are connected to the rope drum. The diameter of the rope drum is 150 mm. What distance must the pads B be at from the center of rotation to cover the same distance as A?A B- Width 50 mm - Width 60 mm- Evidence center 120mm - Construction power 900 N from rotation center.- Maintains a weight of 200 kgwhen the installation force is 1.4kN (μ is missing from the data)M=μF(Ry-Ri)Right answer R=187 mmarrow_forward
- Assume the xy plane is level ground, and that the vertical pole shown in the diagram lies along the z-axis with its base at the origin. If the pole is 5 m tall, and a rope is used to pull on the top of the pole with a force of 400 N as shown, determine the magnitudes of the parallel and perpendicular components of the force vector with respect to the axis of the post i.e. with respect to the z-axis.arrow_forward4-1 Q4: Q5: (20 Marks) Find √48 using False Position Method with three iterations. Hint: the root lies between 3 and 4. (20 Marks)arrow_forwardDetermine the angle between vectors FA and FB that is less than 180 degrees. FA is the vector drawn from the origin to point A (-4, 4, 2) while FB is the vector drawn from the origin to point B (3, 1, -3).arrow_forward
- Find the resultant force vector from adding F1, F2 and F3, where … F1 = {-8i+10j-32k} N F2 is 40 N in magnitude with coordinate direction angles α, β, and γ, of 45, 120 and 60 degrees, respectively and F3 is 22 N in magnitude with transverse and azimuth angles of 65 and 40 degrees, respectively Express your final answer as a Cartesian vector as well as a magnitude with angles.arrow_forwardA 2-kW resistance heater wire with thermal conductivity of k=20 W/mK, a diameter of D=4mm, and a length of L=0.9m is used to boil water. If the outer surface temp of the resistance wire is Ts=110 degrees C, determine the temp at the center of the wire.arrow_forwardA flat-plate solar collector is used to heat water by having water flow through tubes attached at the back of the thin solar absorber plate. The absorber plate has emmisssivity and an absorptivity of 0.9. The top surface where x=0 temp of the absorber is T0=35 degrees C, and solar radiation is incident on the basorber at 500 W/m^2 with a surrounding temp of 0 degrees C. The convection heat transfer coefficient at the absorber surface is 5 W/m^2 K, while the ambient temp is 25 degrees C. Show that the variation of the temp in the basorber plate can be expressed as T(x)=-(q0/k)x+T0, and determine net heat flux, q, absorbed by solar collector.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





