
Thermodynamics: An Engineering Approach
9th Edition
ISBN: 9781259822674
Author: Yunus A. Cengel Dr., Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.5, Problem 129RP
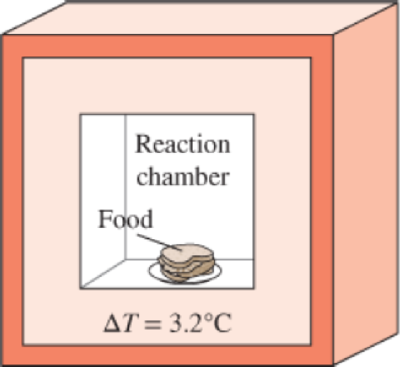
The energy content of a certain food is to be determined in a bomb calorimeter that contains 3 kg of water by burning a 2-g sample of it in the presence of 100 g of air in the reaction chamber. If the water temperature rises by 3.2°C when equilibrium is established, determine the energy content of the food, in kJ/kg, by neglecting the thermal energy stored in the reaction chamber and the energy supplied by the mixer. What is a rough estimate of the error involved in neglecting the thermal energy stored in the reaction chamber?
FIGURE P4–129
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
My answers are incorrect
Picture
What is the weight of a 5-kg substance in N, kN, kg·m/s², kgf, Ibm-ft/s², and lbf?
The weight of a 5-kg substance in N is 49.05
N.
The weight of a 5-kg substance in kN is
KN.
The weight of a 5-kg substance in kg·m/s² is 49.05
kg-m/s².
The weight of a 5-kg substance in kgf is 5.0 kgf.
The weight of a 5-kg substance in Ibm-ft/s² is 11.02 lbm-ft/s².
The weight of a 5-kg substance in lbf is 11.023
lbf.
Chapter 4 Solutions
Thermodynamics: An Engineering Approach
Ch. 4.5 - Is the boundary work associated with...Ch. 4.5 - On a P-V diagram, what does the area under the...Ch. 4.5 - An ideal gas at a given state expands to a fixed...Ch. 4.5 - Calculate the total work, in kJ, for process 13...Ch. 4.5 - Calculate the total work, in Btu, produced by the...Ch. 4.5 - Nitrogen at an initial state of 300 K, 150 kPa,...Ch. 4.5 - The volume of 1 kg of helium in a pistoncylinder...Ch. 4.5 - A pistoncylinder device with a set of stops...Ch. 4.5 - A mass of 5 kg of saturated water vapor at 150 kPa...Ch. 4.5 - A frictionless pistoncylinder device contains 16...
Ch. 4.5 - 1 m3 of saturated liquid water at 200C is expanded...Ch. 4.5 - Argon is compressed in a polytropic process with n...Ch. 4.5 - A gas is compressed from an initial volume of 0.42...Ch. 4.5 - A mass of 1.5 kg of air at 120 kPa and 24C is...Ch. 4.5 - During some actual expansion and compression...Ch. 4.5 - A frictionless pistoncylinder device contains 5 kg...Ch. 4.5 - During an expansion process, the pressure of a gas...Ch. 4.5 - A pistoncylinder device initially contains 0.4 kg...Ch. 4.5 - A pistoncylinder device contains 0.15 kg of air...Ch. 4.5 - Determine the boundary work done by a gas during...Ch. 4.5 - 1 kg of water that is initially at 90C with a...Ch. 4.5 - An ideal gas undergoes two processes in a...Ch. 4.5 - A pistoncylinder device contains 50 kg of water at...Ch. 4.5 - Prob. 26PCh. 4.5 - A closed system like that shown in Fig. P427E is...Ch. 4.5 - A rigid container equipped with a stirring device...Ch. 4.5 - Complete each line of the following table on the...Ch. 4.5 - A substance is contained in a well-insulated rigid...Ch. 4.5 - A 0.5-m3rigid tank contains refrigerant-134a...Ch. 4.5 - A 20-ft3 rigid tank initially contains saturated...Ch. 4.5 - A rigid 10-L vessel initially contains a mixture...Ch. 4.5 - A rigid 1-ft3 vessel contains R-134a originally at...Ch. 4.5 - A pistoncylinder device contains 5 kg of...Ch. 4.5 - A pistoncylinder device contains 0.5 lbm of water...Ch. 4.5 - 2 kg of saturated liquid water at 150C is heated...Ch. 4.5 - An insulated pistoncylinder device contains 5 L of...Ch. 4.5 - A 40-L electrical radiator containing heating oil...Ch. 4.5 - Steam at 75 kPa and 8 percent quality is contained...Ch. 4.5 - A pistoncylinder device initially contains 0.6 m3...Ch. 4.5 - An insulated tank is divided into two parts by a...Ch. 4.5 - Two tanks (Tank A and Tank B) are separated by a...Ch. 4.5 - Is the energy required to heat air from 295 to 305...Ch. 4.5 - A fixed mass of an ideal gas is heated from 50 to...Ch. 4.5 - A fixed mass of an ideal gas is heated from 50 to...Ch. 4.5 - A fixed mass of an ideal gas is heated from 50 to...Ch. 4.5 - Is the relation u = mcv,avgT restricted to...Ch. 4.5 - Is the relation h = mcp,avgT restricted to...Ch. 4.5 - What is the change in the internal energy, in...Ch. 4.5 - Neon is compressed from 100 kPa and 20C to 500 kPa...Ch. 4.5 - What is the change in the enthalpy, in kJ/kg, of...Ch. 4.5 - A mass of 10 g of nitrogen is contained in the...Ch. 4.5 - Determine the internal energy change u of...Ch. 4.5 - Determine the enthalpy change h of oxygen, in...Ch. 4.5 - Is it possible to compress an ideal gas...Ch. 4.5 - Nitrogen in a rigid vessel is cooled by rejecting...Ch. 4.5 - Nitrogen at 100 psia and 300F in a rigid container...Ch. 4.5 - A pistoncylinder device containing carbon-dioxide...Ch. 4.5 - A 3-m3 rigid tank contains hydrogen at 250 kPa and...Ch. 4.5 - 1 kg of oxygen is heated from 20 to 120C....Ch. 4.5 - A 10-ft3 tank contains oxygen initially at 14.7...Ch. 4.5 - A 4-m 5-m 7-m room is heated by the radiator of...Ch. 4.5 - An insulated rigid tank is divided into two equal...Ch. 4.5 - An ideal gas contained in a pistoncylinder device...Ch. 4.5 - A 4-m 5-m 6-m room is to be heated by a...Ch. 4.5 - An insulated pistoncylinder device initially...Ch. 4.5 - Argon is compressed in a polytropic process with n...Ch. 4.5 - An insulated pistoncylinder device contains 100 L...Ch. 4.5 - Air is contained in a variable-load pistoncylinder...Ch. 4.5 - A mass of 15 kg of air in a pistoncylinder device...Ch. 4.5 - Prob. 73PCh. 4.5 - A pistoncylinder device contains 2.2 kg of...Ch. 4.5 - A pistoncylinder device contains 4 kg of argon at...Ch. 4.5 - A spring-loaded pistoncylinder device contains 5...Ch. 4.5 - Prob. 78PCh. 4.5 - Prob. 79PCh. 4.5 - A 1-kg block of iron is heated from 25 to 75C....Ch. 4.5 - The state of liquid water is changed from 50 psia...Ch. 4.5 - During a picnic on a hot summer day, all the cold...Ch. 4.5 - An ordinary egg can be approximated as a...Ch. 4.5 - Consider a 1000-W iron whose base plate is made of...Ch. 4.5 - Stainless steel ball bearings ( = 8085 kg/m3 and...Ch. 4.5 - In a production facility, 1.6-in-thick 2-ft 2-ft...Ch. 4.5 - Long cylindrical steel rods ( = 7833 kg/m3 and cp...Ch. 4.5 - An electronic device dissipating 25 W has a mass...Ch. 4.5 - Prob. 90PCh. 4.5 - Prob. 91PCh. 4.5 - Is the metabolizable energy content of a food the...Ch. 4.5 - Is the number of prospective occupants an...Ch. 4.5 - Prob. 94PCh. 4.5 - Prob. 95PCh. 4.5 - Prob. 96PCh. 4.5 - Consider two identical 80-kg men who are eating...Ch. 4.5 - A 68-kg woman is planning to bicycle for an hour....Ch. 4.5 - A 90-kg man gives in to temptation and eats an...Ch. 4.5 - A 60-kg man used to have an apple every day after...Ch. 4.5 - Consider a man who has 20 kg of body fat when he...Ch. 4.5 - Consider two identical 50-kg women, Candy and...Ch. 4.5 - Prob. 103PCh. 4.5 - Prob. 104PCh. 4.5 - Prob. 105PCh. 4.5 - Prob. 106PCh. 4.5 - Prob. 107PCh. 4.5 - Prob. 108PCh. 4.5 - Prob. 109RPCh. 4.5 - Prob. 110RPCh. 4.5 - Prob. 111RPCh. 4.5 - Prob. 112RPCh. 4.5 - Prob. 113RPCh. 4.5 - Consider a pistoncylinder device that contains 0.5...Ch. 4.5 - Prob. 115RPCh. 4.5 - Air in the amount of 2 lbm is contained in a...Ch. 4.5 - Air is expanded in a polytropic process with n =...Ch. 4.5 - Nitrogen at 100 kPa and 25C in a rigid vessel is...Ch. 4.5 - Prob. 119RPCh. 4.5 - A mass of 3 kg of saturated liquidvapor mixture of...Ch. 4.5 - A mass of 12 kg of saturated refrigerant-134a...Ch. 4.5 - Prob. 122RPCh. 4.5 - A pistoncylinder device contains helium gas...Ch. 4.5 - Prob. 124RPCh. 4.5 - Prob. 125RPCh. 4.5 - Prob. 126RPCh. 4.5 - Prob. 127RPCh. 4.5 - Water is boiled at sea level in a coffeemaker...Ch. 4.5 - The energy content of a certain food is to be...Ch. 4.5 - Prob. 130RPCh. 4.5 - An insulated pistoncylinder device initially...Ch. 4.5 - An insulated rigid tank initially contains 1.4 kg...Ch. 4.5 - In order to cool 1 ton of water at 20C in an...Ch. 4.5 - A 0.3-L glass of water at 20C is to be cooled with...Ch. 4.5 - A well-insulated 3-m 4m 6-m room initially at 7C...Ch. 4.5 - Prob. 137RPCh. 4.5 - Prob. 138RPCh. 4.5 - Prob. 140RPCh. 4.5 - A pistoncylinder device initially contains 0.35 kg...Ch. 4.5 - Two 10-ft3 adiabatic tanks are connected by a...Ch. 4.5 - Prob. 143RPCh. 4.5 - Prob. 144RPCh. 4.5 - A 3-m3 rigid tank contains nitrogen gas at 500 kPa...Ch. 4.5 - A 0.5-m3 rigid tank contains nitrogen gas at 600...Ch. 4.5 - A well-sealed room contains 60 kg of air at 200...Ch. 4.5 - A room contains 75 kg of air at 100 kPa and 15C....Ch. 4.5 - Prob. 149FEPCh. 4.5 - A pistoncylinder device contains 5 kg of air at...Ch. 4.5 - Prob. 151FEPCh. 4.5 - A 2-kW electric resistance heater submerged in 5...Ch. 4.5 - Prob. 153FEPCh. 4.5 - 1.5 kg of liquid water initially at 12C is to be...Ch. 4.5 - Prob. 155FEPCh. 4.5 - An ordinary egg with a mass of 0.1 kg and a...Ch. 4.5 - Prob. 157FEPCh. 4.5 - A 6-pack of canned drinks is to be cooled from 18C...Ch. 4.5 - Prob. 159FEPCh. 4.5 - An ideal gas has a gas constant R = 0.3 kJ/kgK and...Ch. 4.5 - A pistoncylinder device contains an ideal gas. The...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Mych CD 36280 kg. 0.36 givens Tesla truck frailer 2017 Model Vven 96154kph ronge 804,5km Cr Powertrain Across PHVAC rwheel 0.006 0.88 9M² 2 2kW 0.55M ng Zg Prated Trated Pair 20 0.95 1080 kW 1760 Nm 1,2 determine the battery energy required to meet the range when fully loaded determine the approximate time for the fully-loaded truck-trailor to accelerate from 0 to 60 mph while Ignoring vehicle load forcesarrow_forward12-217. The block B is sus- pended from a cable that is at- tached to the block at E, wraps around three pulleys, and is tied to the back of a truck. If the truck starts from rest when ID is zero, and moves forward with a constant acceleration of ap = 0.5 m/s², determine the speed of the block at D the instant x = 2 m. Neglect the size of the pulleys in the calcu- lation. When xƊ = 0, yc = 5 m, so that points C and D are at the Prob. 12-217 5 m yc =2M Xparrow_forwardsolve both and show matlab code auto controlsarrow_forward
- 12-82. The roller coaster car trav- els down the helical path at con- stant speed such that the paramet- ric equations that define its posi- tion are x = c sin kt, y = c cos kt, z = h - bt, where c, h, and b are constants. Determine the mag- nitudes of its velocity and accelera- tion. Prob. 12-82 Narrow_forwardGiven: = refueling Powertran SOURCE EMISSIONS vehide eff eff gasoline 266g co₂/kwh- HEV 0.90 0.285 FLgrid 411ilg Co₂/kWh 41111gCo₂/kWh EV 0.85 0.80 Production 11x10% og CO₂ 13.7 x 10°g CO₂ A) Calculate the breakeven pont (in km driven) for a EV against on HEV in Florida of 0.1kWh/kM Use a drive cycle conversion 5) How efficient would the powertrain of the HEV in this example have to be to break even with an EV in Florida after 150,000 Miles of service (240,000) km Is it plausible to achieve the answer from pert b Consideans the HaXINERY theoretical efficiency of the Carnot cycle is 5020 and there are additional losses of the transMISSION :- 90% efficiency ? c A what do you conclude is the leading factor in why EVs are less emissive than ICE,arrow_forwardsolve autocontrolsarrow_forward
- Problem 3.21P: Air at 100F(38C) db,65F(18C) wb, and sea-level pressure is humidified adiabatically with steam. The steam supplied contains 20 percent moisture(quality of 0.80) at 14.7psia(101.3kpa). The air is humidified to 60 percent relative humidity. Find the dry bulb temperature of the humidified air using (a)chart 1a or 1b and (b) the program PSYCH.arrow_forwardPUNTO 4. calculate their DoF using Gruebler's formula. PUNTO 5. Groundarrow_forwardPUNTO 2. PUNTO 3. calculate their DoF using Gruebler's formula. III IAarrow_forward
- calculate their DoF using Gruebler's formula. PUNTO 6. PUNTO 7. (Ctrl)arrow_forwardA pump delivering 230 lps of water at 30C has a 300-mm diameter suction pipe and a 254-mm diameter discharge pipe as shown in the figure. The suction pipe is 3.5 m long and the discharge pipe is 23 m long, both pipe's materials are cast iron. The water is delivered 16m above the intake water level. Considering head losses in fittings, valves, and major head loss. a) Find the total dynamic head which the pump must supply. b)It the pump mechanical efficiency is 68%, and the motor efficiency is 90%, determine the power rating of the motor in hp.given that: summation of K gate valve = 0.25check valve=390 degree elbow= 0.75foot valve= 0.78arrow_forwardA pump delivering 230 lps of water at 30C has a 300-mm diameter suction pipe and a 254-mm diameter discharge pipe as shown in the figure. The suction pipe is 3.5 m long and the discharge pipe is 23 m long, both pipe's materials are cast iron. The water is delivered 16m above the intake water level. Considering head losses in fittings, valves, and major head loss. a) Find the total dynamic head which the pump must supply. b)It the pump mechanical efficiency is 68%, and the motor efficiency is 90%, determine the power rating of the motor in hp.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=NyOYW07-L5g;License: Standard youtube license