
General, Organic, & Biological Chemistry
3rd Edition
ISBN: 9780073511245
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.60P
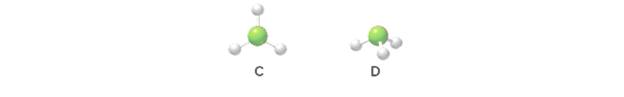
Match each compound with one of the molecular shapes shown below (C or D): (a)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Some of the theories used to describe interface structure can be distinguished by:1. the measured potential difference.2. the distribution of ions in solution.3. the calculation of charge density.4. the external Helmoltz plane.
When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Chapter 4 Solutions
General, Organic, & Biological Chemistry
Ch. 4.1 - Use electron-dot symbols to show how a hydrogen...Ch. 4.1 - Use electron-dot symbols to show how two chlorine...Ch. 4.1 - How many covalent bonds are predicted for each...Ch. 4.1 - Fill in the lone pairs on each atom to give every...Ch. 4.1 - Prob. 4.5PCh. 4.1 - Draw a Lewis structure for each covalent molecule....Ch. 4.1 - Draw a Lewis structure for dimethyl ether (C2H6O)...Ch. 4.2 - Prob. 4.8PCh. 4.2 - Prob. 4.9PCh. 4.2 - Prob. 4.10P
Ch. 4.3 - Prob. 4.11PCh. 4.3 - Prob. 4.12PCh. 4.4 - Prob. 4.13PCh. 4.4 - Draw resonance structures for each polyatomic...Ch. 4.4 - Nitrous oxide, N2O, is a sweet-smelling gas...Ch. 4.5 - Name each compound: (a) CS2; (b) SO2; (c) PCl5;...Ch. 4.5 - Prob. 4.17PCh. 4.5 - What is the shape around the indicated atom in...Ch. 4.6 - NaNH2, sodium amid, is a salt that contains a...Ch. 4.6 - Prob. 4.20PCh. 4.7 - Using the trends in the periodic table, rank the...Ch. 4.7 - Use electronegativity values to classify the...Ch. 4.7 - Prob. 4.23PCh. 4.8 - Prob. 4.24PCh. 4.9 - Prob. 4.25PCh. 4.9 - Prob. 4.26PCh. 4 - For each pair of compounds, classify the bonding...Ch. 4 - For each pair of compounds, classify the bonding...Ch. 4 - Prob. 4.29PCh. 4 - How many bonds and lone pairs are typically...Ch. 4 - Prob. 4.31PCh. 4 - Fill in the lone pairs needed to give the main...Ch. 4 - Prob. 4.33PCh. 4 - Convert the 3-D model of the general anesthetic...Ch. 4 - Draw a valid Lewis structure for each molecule. Hl...Ch. 4 - Draw a valid Lewis structure for each molecule....Ch. 4 - Prob. 4.37PCh. 4 - Prob. 4.38PCh. 4 - Draw a valid Lewis structure for...Ch. 4 - Draw a valid Lewis structure for phosgene, CCl2O ,...Ch. 4 - Draw a valid Lewis structure for each ion: (a)...Ch. 4 - Draw a valid Lewis structure for each ion: (a)...Ch. 4 - Keeping in mind that some elements violate the...Ch. 4 - Keeping in mind that some elements violate the...Ch. 4 - Prob. 4.45PCh. 4 - Prob. 4.46PCh. 4 - Prob. 4.47PCh. 4 - Prob. 4.48PCh. 4 - Prob. 4.49PCh. 4 - Label each pair of compounds are resonance...Ch. 4 - Prob. 4.51PCh. 4 - Draw three resonance structures for the nitrate...Ch. 4 - Name each covalent compound. PBr3 SO3 NCl3 P2S5Ch. 4 - Name each covalent compound. SF6 CBr4 N2O P4O10Ch. 4 - Prob. 4.55PCh. 4 - Prob. 4.56PCh. 4 - Add lone pairs where needed to give octets and...Ch. 4 - Add lone pairs where needed to give octets and...Ch. 4 - Prob. 4.59PCh. 4 - Match each compound with one of the molecular...Ch. 4 - Prob. 4.61PCh. 4 - Add lone pairs where needed to give octets and...Ch. 4 - Prob. 4.63PCh. 4 - Considering each of the given ball-and stick...Ch. 4 - Prob. 4.65PCh. 4 - Prob. 4.66PCh. 4 - Prob. 4.67PCh. 4 - Predict the bond angles around the indicated atoms...Ch. 4 - Prob. 4.69PCh. 4 - Prob. 4.70PCh. 4 - Rank the atoms in each group in order of...Ch. 4 - Prob. 4.72PCh. 4 - Prob. 4.73PCh. 4 - Using electronegativity values, classify the bond...Ch. 4 - Label the bond formed between carbon and each of...Ch. 4 - Label the bond formed between fluroine and each of...Ch. 4 - Which bond in each pair is more polar-that is, has...Ch. 4 - Which bond in each pair is more polar-that is, has...Ch. 4 - Prob. 4.79PCh. 4 - Prob. 4.80PCh. 4 - Label the polar bonds and then decide if each...Ch. 4 - Label the polar bonds and then decide if each...Ch. 4 - Prob. 4.83PCh. 4 - Explain why H2O is a polar molecule but H2S is...Ch. 4 - Convert each ball-and-stick model to a Lewis...Ch. 4 - Convert each ball-and-stick model to a Lewis...Ch. 4 - Answer the following questions about the molecule...Ch. 4 - Answer the following question about the molecule...Ch. 4 - Prob. 4.89PCh. 4 - Lactic acid gives sour milk its distinctive taste....Ch. 4 - Prob. 4.91PCh. 4 - Prob. 4.92PCh. 4 - Prob. 4.93PCh. 4 - Prob. 4.94PCh. 4 - Isobutyl cyanoacrylate is used in medical glues to...Ch. 4 - Prob. 4.96PCh. 4 - Cyclopropane is a stable compound that contains...Ch. 4 - Prob. 4.98CPCh. 4 - Prob. 4.99CPCh. 4 - Prob. 4.100CP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
1. Rub your hands together vigorously. What happens? Discuss the energy transfers and transformations that take...
College Physics: A Strategic Approach (3rd Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
An electric motor has an effective resistance of 32.0 and an inductive reactance of 45.0 when working under l...
Fundamentals of Physics Extended
Whether two metal foil leaves an electroscope get opposite charge when the electroscope is charged.
Physics of Everyday Phenomena
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY