
World of Chemistry
7th Edition
ISBN: 9780618562763
Author: Steven S. Zumdahl
Publisher: Houghton Mifflin College Div
expand_more
expand_more
format_list_bulleted
Question
Chapter 4, Problem 36A
Interpretation Introduction
Interpretation:The name and formula of compounds formed by given cation and anion needs to be determined.
Concept Introduction: An ionic compound is composed of cation and anion. Here cation is positively charged and anion carries negative charge.
The cation and anion can be composed of more than one atoms. Such types of ions are called polyatomic ions.
Expert Solution & Answer
Explanation of Solution
Given information:
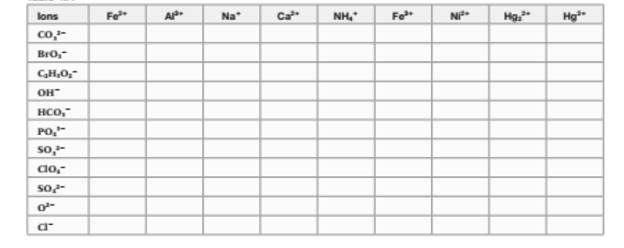
The chemical formula of any compound can be written with criss-cross method in which the valency of both cation and anion are cross multiply to get the simplest whole number of elements in the formula. Thus, the name and formula of compound are listed below;
Ferrous carbonate | Aluminum carbonate | Sodium carbonate | Calcium carbonate | Mercury (I) carbonate | |
iron(II) bromate | Aluminum (III) bromate | Sodium bromate | Calcium bromate | Mercury (II) bromate | |
Iron (II) acetate | Aluminum acetate | Sodium acetate | Calcium acetate | mercury (II) acetate | |
iron (III) hydroxide | Aluminum hydroxide | Sodium hydroxide | Calcium hydroxide | Mercury (II) hydroxide | |
Iron (II) hydrogen carbonate | Aluminum hydrogen carbonate | sodium hydrogen carbonate | calcium hydrogen carbonate | Mercury (II) hydrogen carbonate | |
Iron(II) phosphate | aluminum phosphate | sodium phosphate | calcium phosphate | mercury (II) phosphate | |
iron(II) sulfite | aluminum sulfite | sodium sulfite | calcium sulfite | mercury (II) sulfite | |
iron(II) perchlorate | aluminum perchlorate | sodium perchlorate | calcium perchlorate | Mercury (II) perchlorate | |
iron(II) sulfate | aluminum sulfate | sodium sulfate | calcium sulfate | mercury (II) sulfate | |
iron (II) oxide | aluminum oxide | sodium oxide | calcium oxide | mercury (II) oxide | |
iron (II) chloride | aluminum chloride | sodium chloride | calcium chloride | mercury (II) chloride |
Ammonium carbonate | Ferric carbonate | Nickel carbonate | Mercury (I) carbonate | |
Ammonium bromate | Iron (III) bromate | Nickel (II) bromate | Mercury (I) bromate | |
Ammonium acetate | Iron (III) acetate | Nickel (II) acetate | mercury (I) acetate | |
Ammonium hydroxide | Iron (III) hydroxide | Nickel (II) hydroxide | mercury (I) hydroxide | |
ammonium hydrogen carbonate | iron(III) hydrogen carbonate | nickel (II) hydrogen carbonate | mercury (I) hydrogen carbonate | |
ammonium phosphate | iron(II) phosphate | nickel (II) phosphate | mercury (I) phosphate | |
ammonium sulfite | iron (III) sulfite | Nickel (II) sulfite | Mercury (I) sulfite | |
ammonium perchlorate | iron(III) perchlorate | Nickel(II) perchlorate | Mercury (I) perchlorate | |
ammonium sulfate | iron(III) sulfate | Nickel (II) sulfate | Mercury (I) sulfate | |
Ammonium oxide | iron (III) oxide | Nickel (II) oxide | mercury (I) oxide | |
ammonium chloride | iron (III) chloride | Nickel (II) chloride | mercury (I) chloride |
Conclusion
Criss-cross method is used to find the formula and name of compound by balancing the charges.
Chapter 4 Solutions
World of Chemistry
Ch. 4.1 - Prob. 1RQCh. 4.1 - Prob. 2RQCh. 4.1 - Prob. 3RQCh. 4.1 - Prob. 4RQCh. 4.1 - Prob. 5RQCh. 4.1 - Prob. 6RQCh. 4.2 - Prob. 1RQCh. 4.2 - Prob. 2RQCh. 4.2 - Prob. 3RQCh. 4.2 - Prob. 4RQ
Ch. 4.2 - Prob. 5RQCh. 4.2 - Prob. 6RQCh. 4 - Prob. 1ACh. 4 - Prob. 2ACh. 4 - Prob. 3ACh. 4 - Prob. 4ACh. 4 - Prob. 5ACh. 4 - Prob. 6ACh. 4 - Prob. 7ACh. 4 - Prob. 8ACh. 4 - Prob. 9ACh. 4 - Prob. 10ACh. 4 - Prob. 11ACh. 4 - Prob. 12ACh. 4 - Prob. 13ACh. 4 - Prob. 14ACh. 4 - Prob. 15ACh. 4 - Prob. 16ACh. 4 - Prob. 17ACh. 4 - Prob. 18ACh. 4 - Prob. 19ACh. 4 - Prob. 20ACh. 4 - Prob. 21ACh. 4 - Prob. 22ACh. 4 - Prob. 23ACh. 4 - Prob. 24ACh. 4 - Prob. 25ACh. 4 - Prob. 26ACh. 4 - Prob. 27ACh. 4 - Prob. 28ACh. 4 - Prob. 29ACh. 4 - Prob. 30ACh. 4 - Prob. 31ACh. 4 - Prob. 32ACh. 4 - Prob. 33ACh. 4 - Prob. 34ACh. 4 - Prob. 35ACh. 4 - Prob. 36ACh. 4 - Prob. 37ACh. 4 - Prob. 38ACh. 4 - Prob. 39ACh. 4 - Prob. 40ACh. 4 - Prob. 41ACh. 4 - Prob. 42ACh. 4 - Prob. 43ACh. 4 - Prob. 44ACh. 4 - Prob. 45ACh. 4 - Prob. 46ACh. 4 - Prob. 47ACh. 4 - Prob. 48ACh. 4 - Prob. 49ACh. 4 - Prob. 50ACh. 4 - Prob. 51ACh. 4 - Prob. 1STPCh. 4 - Prob. 2STPCh. 4 - Prob. 3STPCh. 4 - Prob. 4STPCh. 4 - Prob. 5STPCh. 4 - Prob. 6STPCh. 4 - Prob. 7STPCh. 4 - Prob. 8STPCh. 4 - Prob. 9STPCh. 4 - Prob. 10STPCh. 4 - Prob. 11STP
Knowledge Booster
Similar questions
- Identify the mechanism through which the following reaction will proceed and draw the major product. Part 1 of 2 Br KOH EtOH Through which mechanism will the reaction proceed? Select the single best answer. E1 E2 neither Part: 1/2 Part 2 of 2 Draw the major product formed as a result of the reaction. Click and drag to start drawing a structure. Xarrow_forwardWhat is single-point calibration? Provide an example.arrow_forwardDraw the major product formed via an E1 pathway.arrow_forward
- Part 9 of 9 Consider the products for the reaction. Identify the major and minor products. HO Cl The E stereoisomer is the major product and the Z stereoisomer is the minor product ▼ S major product minor productarrow_forwardConsider the reactants below. Answer the following questions about the reaction mechanism and products. HO Clarrow_forwardjulietteyep@gmail.com X YSCU Grades for Juliette L Turner: Orc X 199 A ALEKS - Juliette Turner - Modul X A ALEKS - Juliette Turner - Modul x G butane newman projection - Gox + www-awa.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IBxzaplnN4HsoQggFsejpgqKoyrQrB2dKVAN-BcZvcye0LYa6eXZ8d4vVr8Nc1GZqko5mtw-d1MkNcNzzwZsLf2Tu9_V817y?10Bw7QYjlb il Scribbr citation APA SCU email Student Portal | Main Ryker-Learning WCU-PHARM D MySCU YSCU Canvas- SCU Module 4: Homework (Ch 9-10) Question 28 of 30 (1 point) | Question Attempt: 1 of Unlimited H₂SO heat OH The mechanism of this reaction involves two carbocation intermediates, A and B. Part 1 of 2 KHSO 4 rearrangement A heat B H₂O 2 OH Draw the structure of A. Check Search #t m Save For Later Juliet Submit Assignm 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forward
- The electrons flow from the electron-rich atoms of the nucleophile to the electrons poor atoms of the alkyl halide. Identify the electron rich in the nucleophile. Enter the element symbol only, do not include any changes.arrow_forwardHello, I am doing a court case analysis in my Analytical Chemistry course. The case is about a dog napping and my role is prosecution of the defendant. I am tasked in the Area of Expertise in Neutron Activation and Isotopic Analysis. Attached is the following case study reading of my area of expertise! The landscaping stone was not particularly distinctive in its decoration but matched both the color and pattern of the Fluential’s landscaping stone as well as the stone in the back of the recovered vehicle. Further analysis of the stone was done using a technique called instrumental neutron activation analysis. (Proceed to Neutron Activation data) Photo Notes: Landscaping stone recovered in vehicle. Stone at Fluential’s home is similar inappearance. Finally, the white paint on the brick was analyzed using stable isotope analysis. The brick recovered at the scene had smeared white paint on it. A couple of pieces of brick in the back of the car had white paint on them. They…arrow_forwardCite the stability criteria of an enamine..arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY