
Concept explainers
(a)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles-neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but the number of neutrons is different.
Answer to Problem 25E
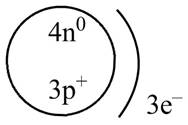
The diagram that represents the arrangement of protons, neutrons, and electrons in
Explanation of Solution
The
The
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
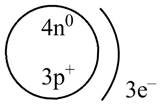
Figure 1
The diagram that represents the arrangement of protons, neutrons, and electrons in
(b)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles-neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but a different number of neutrons.
Answer to Problem 25E
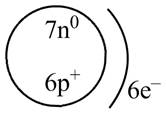
The diagram that represents the arrangement of protons, neutrons, and electrons in
Explanation of Solution
The atomic mass of
The atomic number of
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
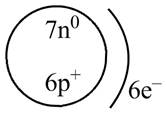
Figure 2
The diagram that represents the arrangement of protons, neutrons, and electrons in
(c)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles-neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but a different number of neutrons.
Answer to Problem 25E
The diagram that represents the arrangement of protons, neutrons, and electrons in
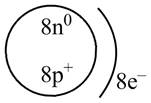
Explanation of Solution
The atomic mass of
The atomic number of
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
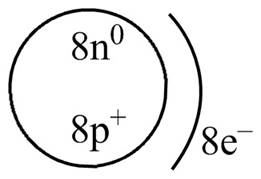
Figure 3
The diagram that represents the arrangement of protons, neutrons, and electrons in
(d)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles-neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but a different number of neutrons.
Answer to Problem 25E
The diagram that represents the arrangement of protons, neutrons, and electrons in
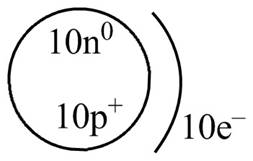
Explanation of Solution
The atomic mass of
The atomic number of
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
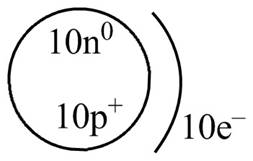
Figure 4
The diagram that represents the arrangement of protons, neutrons, and electrons in
Want to see more full solutions like this?
Chapter 4 Solutions
Introductory Chemistry: Concepts and Critical Thinking (8th Edition)
- Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 85.0899. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forwardUse the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 59 3.0 58 64 43 100 15 23 • You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. + n[] 85 // ? CH4 Previous Nextarrow_forwardWrite the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M* = 128.0632. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 12C 98.90 14N 99.63 160 99.76 Molecular formula 1.008 12.000 14.003 15.995 (In the order CHNO, with no subscripts)arrow_forward
- Can I please get help with this? And can I please the lowest possible significant number?arrow_forwardWhat is the molar mass of a gas that takes three times longer to effuse than helium?arrow_forwardFirst image: I have to show the mecanism (with arows and structures) of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mecanism (with arrows and structures) for the reaction on the left, where the alcohol A is added fast in one portion its not an examarrow_forward
- what is the skeletal structure of a tertiary alkyl fluoride with six carbon atoms and no rings.arrow_forwardOne step of glycolysis is a retro-aldol reaction (aldolase) to produce ATP.Below is the aldol reaction of the equilibrium. Show the mechanism for the base catalyzed reaction. *see imagearrow_forwardProvide the missing information. *see imagearrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





