
Concept explainers
(a)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles, which are neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but a different number of neutrons.
Answer to Problem 26E
The diagram that represents the arrangement of protons, neutrons, and electrons in
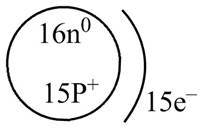
Explanation of Solution
The
The
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
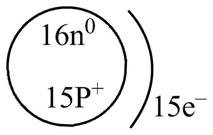
Figure 1
The diagram that represents the arrangement of protons, neutrons, and electrons in
(b)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles, which are neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but a different number of neutrons.
Answer to Problem 26E
The diagram that represents the arrangement of protons, neutrons, and electrons in
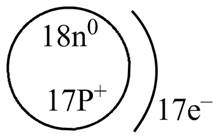
Explanation of Solution
The atomic mass of
The atomic number of
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
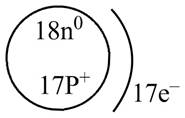
Figure 2
The diagram that represents the arrangement of protons, neutrons, and electrons in
(c)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles, which are neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but a different number of neutrons.
Answer to Problem 26E
The diagram that represents the arrangement of protons, neutrons, and electrons in
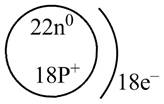
Explanation of Solution
The atomic mass of
The atomic number of
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
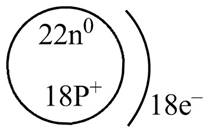
Figure 3
The diagram that represents the arrangement of protons, neutrons, and electrons in
(d)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles-neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but a different number of neutrons.
Answer to Problem 26E
The diagram that represents the arrangement of protons, neutrons, and electrons in
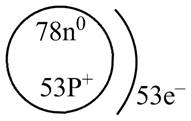
Explanation of Solution
The atomic mass of
The atomic number of
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
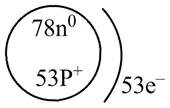
Figure 4
The diagram that represents the arrangement of protons, neutrons, and electrons in
Want to see more full solutions like this?
Chapter 4 Solutions
Introductory Chemistry: Concepts and Critical Thinking (8th Edition)
- Write "most" under the member of each trio which is most stable. Write "least under the member of each trio which is least stable. b) Draw a Fischer projection of a pair of enantiomers with three chiral carbons. Which of these two would you expect to be more soluble in water? Why? 1-butanol 1-heptanol Which of these two would you expect to have the higher boiling point? Why? hexyl methyl ether 1-heptanolarrow_forwardWrite "most" under the most acidic compound. Write "least" under the least acidic compound. OH NO₂ OCH3 Br 9. Compound X, C50H84F2, reacts with excess H2/Pd to give a C50H88F2 compound. How many rings are in X? How many double bonds are in X? Show your work.arrow_forward4. State whether these two are: a) the same molecule b) c) d) different compounds that are not isomers constitutional isomers diastereomers e) enantiomers CH3 CH₁₂ H OH HO H H OH HO H CH, CH₂ 5. a) How many stereocenters does this compound have? b) How many stereoisomers are possible for this compound? CH₂ OH CHCHarrow_forward
- Calculating the pH at equivalence of a titration A chemist titrates 210.0 mL of a 0.1003 M hydrobromic acid (HBr) solution with 0.7550M KOH solution at 25 °C. Calculate the pH at equivalence. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. pH = ] ☑ o0o 18 Ararrow_forwardDo you do chemistry assignmentsarrow_forwardUsing the conditions of spontaneity to deduce the signs of AH and AS Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A This reaction is always spontaneous, but proceeds slower at temperatures above 120. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 117. °C. AS is (pick one) ΔΗ is (pick one) This reaction is slower below 20. °C than C above. AS is |(pick one) ? 18 Ar 1arrow_forward
- Calculating the pH at equivalence of a titration Try Again Your answer is incorrect. 0/5 a A chemist titrates 70.0 mL of a 0.7089 M hydrocyanic acid (HCN) solution with 0.4574M KOH solution at 25 °C. Calculate the pH at equivalence. The pK of hydrocyanic acid is 9.21. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. pH = 11.43] G 00. 18 Ar B•arrow_forwardBiological Macromolecules Naming and drawing the products of aldose oxidation and reduction aw a Fischer projection of the molecule that would produce L-ribonic acid if it were subjected to mildly oxidizing reaction conditions. Click and drag to start drawing a structure. X AP ‡ 1/5 Naor Explanation Check McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibilarrow_forward● Biological Macromolecules Identifying the parts of a disaccharide Take a look at this molecule, and then answer the questions in the table below it. CH2OH O H H H OH OH OH H H CH2OH H O OH H OH H H H H OH Is this a reducing sugar? Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. Explanation Check O yes X O no ○ yes O no Uarrow_forward
- The aim of the lab is to measure the sodium content from tomato sauce using the Mohr titration method. There are two groups being: Regular Tomato sauce & Salt Reduced tomato sauce QUESTION: State how you would prepare both Regular & Salt reduced tomato sauce samples for chemical analysis using the Mohr titration methodarrow_forwardUsing the conditions of spontaneity to deduce the signs of AH and AS Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds faster at temperatures above -48. °C. ΔΗ is (pick one) ✓ AS is (pick one) B This reaction is spontaneous except below 114. °C but proceeds at a slower rate below 135. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is C This reaction is exothermic and proceeds faster at temperatures above -43. °C. (pick one) AS is (pick one) v Х 5 ? 18 Ararrow_forwardion. A student proposes the following Lewis structure for the perchlorate (CIO) io : :0: : Cl : - - : :0: ك Assign a formal charge to each atom in the student's Lewis structure. atom central O formal charge ☐ top O ☐ right O ☐ bottom O ☐ Cl ☐arrow_forward
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





