
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.3, Problem 17P
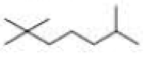
What is each compound’s systematic name?
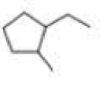
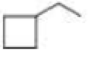
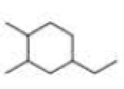
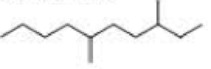
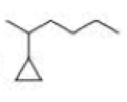
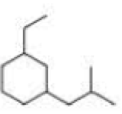
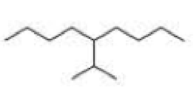
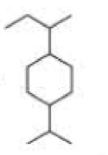
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Design experiments in UV-Vis to figure the optimal mole ratio of copper (1:1, 2:1, 3:1 and etc)versus ethambutol using all necessary chemicals including dihydrochloride and copper nitrate hemipentahydrate and sodium hydroxide. Show how UV-Vis absorbance and maximum wavelength would change in response
Correct each molecule in the drawing area below so that it has the condensed structure it would have if it were dissolv
a 0.1 M aqueous solution of HCI.
If there are no changes to be made, check the No changes box under the drawing area.
No changes.
HO—CH,—C—CH,—OH
X
5
2
2
2
HO–CH,—CH,—C—CH,—OH
Explanation
Check
Center Accessi
©2025 on
5
C
Make the calculations to prepare 2M H2SO4, from concentrated H2SO4 (98%; density: 1.84 g/mL).
Chapter 3 Solutions
Organic Chemistry
Ch. 3.1 - Name each of the following:Ch. 3.1 - Draw the structure of a compound with molecular...Ch. 3.1 - Draw the structures and name the four...Ch. 3.1 - Prob. 6PCh. 3.1 - Draw the structure for each of the following: a....Ch. 3.1 - Name the following compounds: a. CH3OCH2CH3 b....Ch. 3.2 - Prob. 11PCh. 3.2 - Prob. 12PCh. 3.3 - Prob. 13PCh. 3.3 - Prob. 14P
Ch. 3.3 - Prob. 15PCh. 3.3 - Prob. 16PCh. 3.3 - What is each compounds systematic name?Ch. 3.4 - Give two names for each of the following alkyl...Ch. 3.4 - Prob. 19PCh. 3.5 - a. What is each ethers systematic name? 1....Ch. 3.6 - Draw the structures of a homologous series of...Ch. 3.6 - Give each of the following a systematic name and...Ch. 3.6 - Prob. 23PCh. 3.6 - Prob. 24PCh. 3.7 - Are the following compounds primary, secondary, or...Ch. 3.7 - Prob. 26PCh. 3.7 - Prob. 27PCh. 3.7 - For each of the following, give the systematic...Ch. 3.8 - Predict the approximate size of the following bond...Ch. 3.9 - Prob. 30PCh. 3.9 - Prob. 31PCh. 3.9 - Prob. 32PCh. 3.9 - Rank the following compounds from highest boiling...Ch. 3.9 - Rank the compounds in each set from highest...Ch. 3.9 - Prob. 35PCh. 3.9 - In which solvent would cyclohexane have the lowest...Ch. 3.10 - a. Draw all the staggered and eclipsed conformers...Ch. 3.10 - Prob. 38PCh. 3.10 - Using Newman projections, draw the most stable...Ch. 3.11 - The bond angles in a regular polygon with n sides...Ch. 3.11 - Prob. 41PCh. 3.11 - Prob. 42PCh. 3.12 - Draw 1,2,3,4,5,6-hexachlorocydohexane with a. all...Ch. 3.13 - The chair conformer of fluorocyclohexane is 0.25...Ch. 3.13 - Using the data in Table 3.9, calculate the...Ch. 3.14 - Prob. 46PCh. 3.14 - Which has a higher percentage of the...Ch. 3.14 - For each of the following disubstituted...Ch. 3.14 - a. Calculate the energy difference between the two...Ch. 3 - a. How many hydrogen does an alkene with 17...Ch. 3 - Draw the structure of octane and isooctaneCh. 3 - Draw a condensed structure and a skeletal...Ch. 3 - Prob. 53PCh. 3 - a. What is each compounds systematic name? b. Draw...Ch. 3 - Which of the following represents a cis isomer?Ch. 3 - a. How many primary carbons does each of the...Ch. 3 - Which of the following conformers of isobutyl...Ch. 3 - Draw a skeletal structure for an alkane that has...Ch. 3 - What is each compounds systematic name? a....Ch. 3 - Which bus a. the higher boiling point:...Ch. 3 - a. Draw Newman projections of the two conformers...Ch. 3 - Ansaid and Motrin belong to the group of drugs...Ch. 3 - Prob. 63PCh. 3 - A student was given the structural formulas of...Ch. 3 - Which of the following conformers has the highest...Ch. 3 - Prob. 66PCh. 3 - Prob. 67PCh. 3 - For rotation about the C-3C-4 bond of...Ch. 3 - Prob. 69PCh. 3 - What is each compounds systematic name? a. b. c....Ch. 3 - Prob. 71PCh. 3 - Why are lower molecular weight alcohols more...Ch. 3 - a. Draw a potential energy diagram for rotation...Ch. 3 - For each of the following compound, determine...Ch. 3 - How many ethers have molecular formula C5H12O?...Ch. 3 - Draw the most stable conformer of the following...Ch. 3 - What is each compounds systematic name?Ch. 3 - Calculate the energy difference between the two...Ch. 3 - The most stable from of glucose (blood sugar) is a...Ch. 3 - Prob. 80PCh. 3 - Explain the following: a. 1-Hexanol has a higher...Ch. 3 - One of the chair conformers of cis-...Ch. 3 - Bromine is a larger atom than chlorine, but the...Ch. 3 - Name the following compounds:Ch. 3 - Prob. 85PCh. 3 - Using the data obtained in Problem 85, calculate...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H CH3 CH3 b) Write the products of your compound and the following reagents. If the reaction would not work for your compound, write "no reaction" and explain the problem. NaCN H* H₂NNHCH5 H* -à NaBH -à CH2MgBr Cro₁₂ --à H3O+ -à c) Would your compound give a positive Tollen's test? Why or why not?arrow_forwardHomework 4 Chem 204 Dr. Hellwig Consider this compound, which will be referred to as "your compound". a) Name your compound according to the IUPAC system. Include stereochemistry (E/Z/R/S) H CH3 CH3arrow_forwardWhat is the mechanism for this?arrow_forward
- 21.50 Determine the combinations of haloalkane(s) and alkoxide(s) that could be used to synthesize the following ethers through Williamson ether synthesis. (a) (c) (d) (e) (f) H₂COarrow_forward1. Arrange the following in order of increasing bond energy (lowest bond energy first, highest bond energy last). Provide your rationale. C=C, C-F, C=C, C-N, C-C List the bond order for each example.arrow_forwardWhat is the major enolate formed when treated with LDA? And why that one?arrow_forward
- 4. Calculate the total number of sigma bonds and total number of pi bonds in each of the following compounds. a. HH :D: +1 I H-N-C-C-O-H I H b. HH H Н :N=C-C-C=C-CEC-H :0: total o H-C-H H-C = `C-H I H. 11 H-C = C= CH H total o total π total π 1 Harrow_forwardIn the following reaction, what quantity in moles of CH₃OH are required to give off 4111 kJ of heat? 2 CH₃OH (l) + 3 O₂ (g) → 2 CO₂ (g) + 4 H₂O(g) ∆H° = -1280. kJarrow_forwardIndicate the processes in the dismutation of Cu2O.arrow_forward
- 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. 2600 2400 2200 2000 1800 1600 1400 1200 1000 800 Potential Energy (kJ) 600 400 200 0 -200- -400 -600- -800 (i) Cl₂ (g) + Pt(s) → 2Cl (g) + Pt(s) (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) Ea = 1550 kJ Ea = 2240 kJ (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2350 kJ AH=-950 kJ ΔΗ = 575 ΚΙ AH=-825 kJ a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ Reaction Progress b. What is the overall chemical equation? c. What is the overall change in enthalpy for the above chemical reaction? d. What is the overall amount of activation energy for the above chemical reaction? e. Which reaction intermediate would be considered a catalyst (if any) and why? f. If you were to add 2700kJ of energy to the reaction (e.g. 2700 kl of heat or electricity), would you be able to make the reaction reverse itself (i.e. have…arrow_forwarddraw the enolate anion and the carbonyl that would be needed to make this product through an aldol addition reaction.arrow_forwardDraw the Michael Adduct and the final product of the Robinson annulation reaction. Ignore inorganic byproducts.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY