
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 77P
What is each compound’s systematic name?
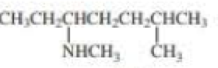
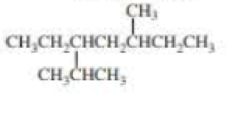
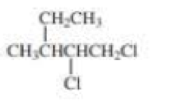
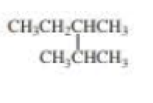
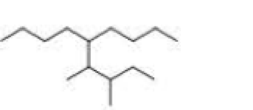
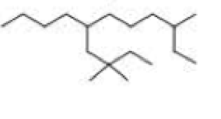
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
11
Organic Chemistry
Organic Nomenclature Practice
Name/Functional Group
n-butane
Formula
Structural Formula
(1) C4tt10
H3C
C-
(2) CH3CH2CH2 CH 3
H₂
-CH3
Н2
name & functional group
(1) and (2)
OH
H₁₂C
Н2
name only
(1) and (2)
name only
(1) and (2)
H₁C - = - CH₂
Н2
HC=C-C
CH3
Under aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to
form the final product 2:
NC
H₂O
он-
H₂O
1
2
OH
Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like.
Click and drag to start drawing a
structure.
Assign these COSY Spectrum
Chapter 3 Solutions
Organic Chemistry
Ch. 3.1 - Name each of the following:Ch. 3.1 - Draw the structure of a compound with molecular...Ch. 3.1 - Draw the structures and name the four...Ch. 3.1 - Prob. 6PCh. 3.1 - Draw the structure for each of the following: a....Ch. 3.1 - Name the following compounds: a. CH3OCH2CH3 b....Ch. 3.2 - Prob. 11PCh. 3.2 - Prob. 12PCh. 3.3 - Prob. 13PCh. 3.3 - Prob. 14P
Ch. 3.3 - Prob. 15PCh. 3.3 - Prob. 16PCh. 3.3 - What is each compounds systematic name?Ch. 3.4 - Give two names for each of the following alkyl...Ch. 3.4 - Prob. 19PCh. 3.5 - a. What is each ethers systematic name? 1....Ch. 3.6 - Draw the structures of a homologous series of...Ch. 3.6 - Give each of the following a systematic name and...Ch. 3.6 - Prob. 23PCh. 3.6 - Prob. 24PCh. 3.7 - Are the following compounds primary, secondary, or...Ch. 3.7 - Prob. 26PCh. 3.7 - Prob. 27PCh. 3.7 - For each of the following, give the systematic...Ch. 3.8 - Predict the approximate size of the following bond...Ch. 3.9 - Prob. 30PCh. 3.9 - Prob. 31PCh. 3.9 - Prob. 32PCh. 3.9 - Rank the following compounds from highest boiling...Ch. 3.9 - Rank the compounds in each set from highest...Ch. 3.9 - Prob. 35PCh. 3.9 - In which solvent would cyclohexane have the lowest...Ch. 3.10 - a. Draw all the staggered and eclipsed conformers...Ch. 3.10 - Prob. 38PCh. 3.10 - Using Newman projections, draw the most stable...Ch. 3.11 - The bond angles in a regular polygon with n sides...Ch. 3.11 - Prob. 41PCh. 3.11 - Prob. 42PCh. 3.12 - Draw 1,2,3,4,5,6-hexachlorocydohexane with a. all...Ch. 3.13 - The chair conformer of fluorocyclohexane is 0.25...Ch. 3.13 - Using the data in Table 3.9, calculate the...Ch. 3.14 - Prob. 46PCh. 3.14 - Which has a higher percentage of the...Ch. 3.14 - For each of the following disubstituted...Ch. 3.14 - a. Calculate the energy difference between the two...Ch. 3 - a. How many hydrogen does an alkene with 17...Ch. 3 - Draw the structure of octane and isooctaneCh. 3 - Draw a condensed structure and a skeletal...Ch. 3 - Prob. 53PCh. 3 - a. What is each compounds systematic name? b. Draw...Ch. 3 - Which of the following represents a cis isomer?Ch. 3 - a. How many primary carbons does each of the...Ch. 3 - Which of the following conformers of isobutyl...Ch. 3 - Draw a skeletal structure for an alkane that has...Ch. 3 - What is each compounds systematic name? a....Ch. 3 - Which bus a. the higher boiling point:...Ch. 3 - a. Draw Newman projections of the two conformers...Ch. 3 - Ansaid and Motrin belong to the group of drugs...Ch. 3 - Prob. 63PCh. 3 - A student was given the structural formulas of...Ch. 3 - Which of the following conformers has the highest...Ch. 3 - Prob. 66PCh. 3 - Prob. 67PCh. 3 - For rotation about the C-3C-4 bond of...Ch. 3 - Prob. 69PCh. 3 - What is each compounds systematic name? a. b. c....Ch. 3 - Prob. 71PCh. 3 - Why are lower molecular weight alcohols more...Ch. 3 - a. Draw a potential energy diagram for rotation...Ch. 3 - For each of the following compound, determine...Ch. 3 - How many ethers have molecular formula C5H12O?...Ch. 3 - Draw the most stable conformer of the following...Ch. 3 - What is each compounds systematic name?Ch. 3 - Calculate the energy difference between the two...Ch. 3 - The most stable from of glucose (blood sugar) is a...Ch. 3 - Prob. 80PCh. 3 - Explain the following: a. 1-Hexanol has a higher...Ch. 3 - One of the chair conformers of cis-...Ch. 3 - Bromine is a larger atom than chlorine, but the...Ch. 3 - Name the following compounds:Ch. 3 - Prob. 85PCh. 3 - Using the data obtained in Problem 85, calculate...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Assign these C-NMR and H-NMR Spectrumarrow_forwardPredict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forwardPredict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forward
- Predict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forward
- Predict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forward
- Predict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forwardPredict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? H R+ H2O Δ OH 0= CH3-CH-O-CH3 + CH3-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. dyarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY