
Concept explainers
Complete and balance the equations below, and classify them as precipitation, acid-base, gas-forming, or
(a) NiCO3 + H2SO4 →.
(b) Co(OH)2 + HBr →
(c) AgCH3CO2 + NaCI →
(d) NiO + CO →.
(a)
Interpretation:
- The given reaction has to be classified as precipitation or acid-base or gas forming reaction and the reaction has to be balanced.
- State of the product and reactant should be shown.
Concept introduction:
Precipitation reaction: The formation of the product is insoluble when the ions combine in the solution is called precipitation reaction.
Acid - base reaction: Formation of the salt from the cation from the base and anion from the acid and formation of water is also the product.
Gas forming reaction: The reaction of acid and metal carbonates which produce carbonic acid. The carbonic acid decomposes which gives water and carbon dioxide.
Oxidation - reduction reaction: The electrons are transferred to one to other is called oxidation reduction reaction.
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of
Almost all the salts of
Salts of F- are soluble. But some of the fluoride salt of
Salts of
Insoluble compounds in water:
Most of the salts of
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides,
Answer to Problem 64PS
The given reaction is gas forming reaction and the balanced equation is shown below.
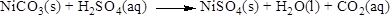
Explanation of Solution
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is gas forming reaction and the balanced equation is shown below
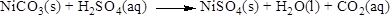
The given compound is nickel carbonate and sulfuric acid. In this reaction nickel carbonate reaction with sulfuric acid to give nickel sulfate and carbon dioxide and water.
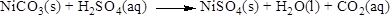
Balance the equation,
The reaction is already balanced. Therefore the balanced equation is given below.
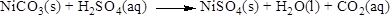
(b)
Interpretation:
- The given reaction has to be classified as precipitation or acid-base or gas forming reaction and the reaction has to be balanced.
- State of the product and reactant should be shown.
Concept introduction:
Precipitation reaction: The formation of the product is insoluble when the ions combine in the solution is called precipitation reaction.
Acid - base reaction: Formation of the salt from the cation from the base and anion from the acid and formation of water is also the product.
Gas forming reaction: The reaction of acid and metal carbonates which produce carbonic acid. The carbonic acid decomposes which gives water and carbon dioxide.
Oxidation - reduction reaction: The electrons are transferred to one to other is called oxidation reduction reaction.
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of
Almost all the salts of
Salts of F- are soluble. But some of the fluoride salt of
Salts of
Insoluble compounds in water:
Most of the salts of
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides,
Answer to Problem 64PS
The given reaction is acid - base reaction and the state of the reaction is shown below.

Explanation of Solution
The given reaction is acid - base reaction and the state of the reaction is shown below

The given compound is Cobalt(II) hydroxide and hydrogen bromide. In this reaction Cobalt(II) hydroxide reaction with hydrogen bromide to give cobalt(II) bromide and formation of water is also the product.
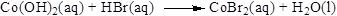
Balance the equation,
Balance the bromine atom in the given equation, when balancing the equation, we should not alter the subscripts and we can change coefficients. There are two bromine atoms in the right side and one bromine atoms in the left side. Therefore two molecule of hydrogen bromide is added to left side of reaction. Therefore the balanced equation is given below.
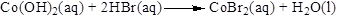
Balance the hydrogen atom in the given equation. There are four hydrogen atoms in the left side and two hydrogen atoms in the right side. Therefore two molecule of water is added to right side of reaction. Therefore the balanced equation is given below.

(c)
Interpretation:
- The given reaction has to be classified as precipitation or acid-base or gas forming reaction and the reaction has to be balanced.
- State of the product and reactant should be shown.
Concept introduction:
Precipitation reaction: The formation of the product is insoluble when the ions combine in the solution is called precipitation reaction.
Acid - base reaction: Formation of the salt from the cation from the base and anion from the acid and formation of water is also the product.
Gas forming reaction: The reaction of acid and metal carbonates which produce carbonic acid. The carbonic acid decomposes which gives water and carbon dioxide.
Oxidation - reduction reaction: The electrons are transferred to one to other is called oxidation reduction reaction.
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of
Almost all the salts of
Salts of F- are soluble. But some of the fluoride salt of
Salts of
Insoluble compounds in water:
Most of the salts of
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides,
Answer to Problem 64PS
The given reaction is precipitation reaction and the state of the reaction is shown below

Explanation of Solution
The given reaction is precipitation reaction and the state of the reaction is shown below

The given compound is silver acetate and sodium chloride. In this reaction silver acetate reaction with sodium chloride to give sodium acetate and silver chloride. Almost all the salts of

Balance the equation,
The reaction is already balanced. Therefore the balanced equation is given below.

(d)
Interpretation:
- The given reaction has to be classified as precipitation or acid-base or gas forming reaction and the reaction has to be balanced.
- State of the product and reactant should be shown.
Concept introduction:
Precipitation reaction: The formation of the product is insoluble when the ions combine in the solution is called precipitation reaction.
Acid - base reaction: Formation of the salt from the cation from the base and anion from the acid and formation of water is also the product.
Gas forming reaction: The reaction of acid and metal carbonates which produce carbonic acid. The carbonic acid decomposes which gives water and carbon dioxide.
Oxidation - reduction reaction: The electrons are transferred to one to other is called oxidation reduction reaction.
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of
Almost all the salts of
Salts of F- are soluble. But some of the fluoride salt of
Salts of
Insoluble compounds in water:
Most of the salts of
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides,
Answer to Problem 64PS
The given reaction is oxidation and reduction reaction and the state of the reaction is shown below.

Explanation of Solution
The given reaction is oxidation and reduction reaction and the state of the reaction is shown below

The given compound is nickel oxide and carbon monoxide. In this reaction nickel oxide reaction with carbon monoxide to give nickel and carbon dioxide gas. Here the oxidation state of nickel is

Balance the equation,
The reaction is already balanced. Therefore the balanced equation is given below.

Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry & Chemical Reactivity
- Question: Find both the b (gradient) and a (y-intercept) value from the list of data below: (x1 -x̄) 370.5 (y1 - ȳ) 5.240 (x2 - x̄) 142.5 (y2 - ȳ) 2.004 (x3 - x̄) 28.5 (y3 - ȳ) 0.390 (x4 - x̄) -85.5 (y4 - ȳ) -1.231 (x5 - x̄) -199.5 (y5 - ȳ) -2.829 (x6 - x̄) -256.5 (y6 - ȳ) -3.575arrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3Cu+ (aq) + Cro²¯ (aq) +4H₂O (1) → 3Cu²+ (aq) +Cr(OH)3 (s)+5OH˜¯ (aq) 0 kJ ☐ x10 00. 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 241.7 mL of a 0.4900M solution of methylamine (CH3NH2) with a 0.7800M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 17.7 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☑ ? 18 Ararrow_forward
- The following is two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0mmol/L 262.7mmol/L QUESTION: For both groups (Regular & Salt Reduced tomato sauce) of data provide answers to the following calculations below: 1. Standard Deviation (Sx) 2. T Values (t0.05,4) 3. 95% Confidence Interval (mmol/L) 4. [Na+] (mg/100 mL) 5. 95% Confidence Interval (mg/100 mL)arrow_forwardIf we have leucine (2-amino-4-methylpentanoic acid), alanine (2-aminopropanoic acid) and phenylalanine (2-amino-3-phenylpropanoic acid), indicate the tripeptides that can be formed (use the abbreviated symbols Leu., Ala and Phe).arrow_forward
- Briefly state why trifluoroacetic acid is more acidic than acetic acid.arrow_forwardExplain why acid chlorides are more reactive than amides in reactions with nucleophiles.arrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 101.7 mL of a 0.3500M solution of piperidine (C5H10NH) with a 0.05700M solution of HClO4. The pK of piperidine is 2.89. Calculate the pH of the base solution after the chemist has added 682.9 mL of the HClO solution to it. 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO solution added. 4 Round your answer to 2 decimal places. pH = .11 00. 18 Ararrow_forward
- The following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0 262.7 QUESTION: For both groups of data provide answers to the calculations attached in the imagearrow_forward7. Concentration and uncertainty in the estimate of concentration (class data) Class mean for sample (Regular) |[Cl-] (mmol/L) class mean Sn za/2 95% Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Confidence Interval (mg/100 mL)arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





