
Campbell Biology in Focus (2nd Edition)
2nd Edition
ISBN: 9780321962751
Author: Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Jane B. Reece
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 2TYU
Which
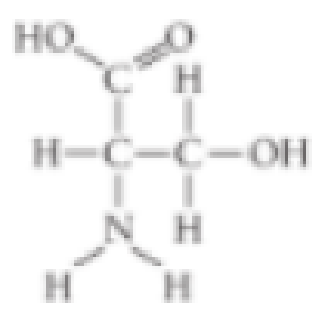
- A. carboxyl
- B. sulfhydryl
- C. hydroxyl
- D. amino
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please refer below
AaBbCc X AaBbCc individuals are crossed.
What is the probability of their offspring having a genotype AABBCC?
circle a nucleotide in the image
Chapter 3 Solutions
Campbell Biology in Focus (2nd Edition)
Ch. 3.1 - How are gasoline and fat chemically similar?Ch. 3.1 - Which molecules in Figure 3.4a re isomers? For...Ch. 3.1 - Prob. 3CCCh. 3.1 - Prob. 4CCCh. 3.2 - How many molecules of water are needed to...Ch. 3.2 - WHAT IF? Suppose you eat a serving of fish. What...Ch. 3.3 - Write the formula for a monosaccharide that has...Ch. 3.3 - A dehydration reaction joins two glucose molecules...Ch. 3.3 - WHAT IF? After a cow is given antibiotics to treat...Ch. 3.4 - Compare the structure of a fat (triglyceride) with...
Ch. 3.4 - Why are human sex hormones considered lipids?Ch. 3.4 - Prob. 3CCCh. 3.5 - Why does a denatured protein no longer function...Ch. 3.5 - What parts of a polypeptide participate in the...Ch. 3.5 - WHAT IF? Where would you expect a polypeptide...Ch. 3.6 - DRAW IT Go to Figure 3.27a and, for the top three...Ch. 3.6 - Prob. 2CCCh. 3.7 - How would sequencing the entire genome of an...Ch. 3.7 - Given the function of DNA, why would you expect...Ch. 3 - Prob. 1TYUCh. 3 - Which functional group is not present in this...Ch. 3 - MAKE CONNECTIONS Which chemical group is most...Ch. 3 - Prob. 4TYUCh. 3 - Which of the following statements concerning...Ch. 3 - The structural level of a protein least a fleeted...Ch. 3 - Enzymes that break down DNA catalyze the...Ch. 3 - Prob. 8TYUCh. 3 - The molecular formula for glucose is C6H12O6. What...Ch. 3 - Construct a table that organizes the following...Ch. 3 - Prob. 11TYUCh. 3 - Prob. 12TYUCh. 3 - FOCUS ON ORGANIZATION Proteins, which have diverse...Ch. 3 - Prob. 14TYUCh. 3 - SYNTHESIZE YOUR KNOWLEDGE Given that the function...
Additional Science Textbook Solutions
Find more solutions based on key concepts
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
1. Rub your hands together vigorously. What happens? Discuss the energy transfers and transformations that take...
College Physics: A Strategic Approach (3rd Edition)
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- "One of the symmetry breaking events in mouse gastrulation requires the amplification of Nodal on the side of the embryo opposite to the Anterior Visceral Endoderm (AVE). Describe one way by which Nodal gets amplified in this region." My understanding of this is that there are a few ways nodal is amplified though I'm not sure if this is specifically occurs on the opposite side of the AVE. 1. pronodal cleaved by protease -> active nodal 2. Nodal -> BMP4 -> Wnt-> nodal 3. Nodal-> Nodal, Fox1 binding site 4. BMP4 on outside-> nodal Are all of these occuring opposite to AVE?arrow_forwardIf four babies are born on a given day What is the chance all four will be girls? Use genetics lawsarrow_forwardExplain each punnet square results (genotypes and probabilities)arrow_forward
- Give the terminal regression line equation and R or R2 value: Give the x axis (name and units, if any) of the terminal line: Give the y axis (name and units, if any) of the terminal line: Give the first residual regression line equation and R or R2 value: Give the x axis (name and units, if any) of the first residual line : Give the y axis (name and units, if any) of the first residual line: Give the second residual regression line equation and R or R2 value: Give the x axis (name and units, if any) of the second residual line: Give the y axis (name and units, if any) of the second residual line: a) B1 Solution b) B2 c)hybrid rate constant (λ1) d)hybrid rate constant (λ2) e) ka f) t1/2,absorb g) t1/2, dist h) t1/2, elim i)apparent central compartment volume (V1,app) j) total AUC (short cut method) k) apparent volume of distribution based on AUC (VAUC,app) l)apparent clearance (CLapp) m) absolute bioavailability of oral route (need AUCiv…arrow_forwardYou inject morpholino oligonucleotides that inhibit the translation of follistatin, chordin, and noggin (FCN) at the 1 cell stage of a frog embryo. What is the effect on neurulation in the resulting embryo? Propose an experiment that would rescue an embryo injected with FCN morpholinos.arrow_forwardParticipants will be asked to create a meme regarding a topic relevant to the department of Geography, Geomatics, and Environmental Studies. Prompt: Using an online art style of your choice, please make a meme related to the study of Geography, Environment, or Geomatics.arrow_forward
- Plekhg5 functions in bottle cell formation, and Shroom3 functions in neural plate closure, yet the phenotype of injecting mRNA of each into the animal pole of a fertilized egg is very similar. What is the phenotype, and why is the phenotype so similar? Is the phenotype going to be that there is a disruption of the formation of the neural tube for both of these because bottle cell formation is necessary for the neural plate to fold in forming the neural tube and Shroom3 is further needed to close the neural plate? So since both Plekhg5 and Shroom3 are used in forming the neural tube, injecting the mRNA will just lead to neural tube deformity?arrow_forwardWhat are some medical issues or health trends that may have a direct link to the idea of keeping fat out of diets?arrow_forwardwhat did charles darwin do in sciencearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Cengage Learning


Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license