
Concept explainers
Three processes that have been used for the industrial manufacture of acrylonitrile (CH2CHCN), an important chemical used in the manufacture of plastics, synthetic rubber, and fibers, are shown below. Use bond energy values (Table 3-3) to estimate ∆E for each of the reactions.
a. 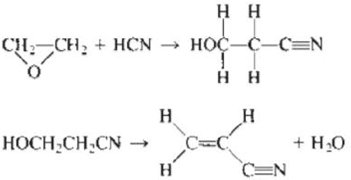
b. 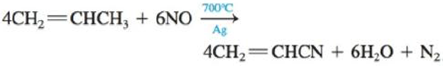
The nitrogen-oxygen bond energy in nitric oxide (NO) is 630. kJ/mol.
c. 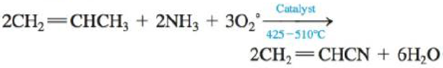
(a)
Interpretation: The change in energy for the given chemical reactions has to be calculated.
Concept introduction: In a chemical reaction, energy is gained, endothermic reactions, or released, exothermic reactions. The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
To determine: The change in energy for the stated reactions.
Answer to Problem 152CP
The required energy change is
Explanation of Solution
Given
The chemical reaction involved is,
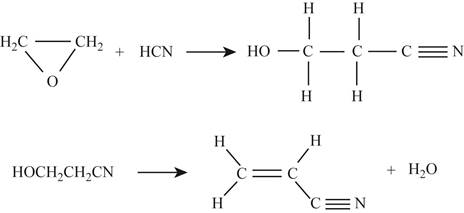
Figure 1
Formula
In the first reaction,
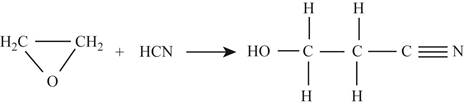
Figure 2
For first reactant,
Hence, the total energy required
For
Hence, the total energy required
Now, the total energy required for the reactants combined
Product bonds,
Hence,
The total energy released when the product is formed
So the change in energy for the first reaction is,
In the second reaction,
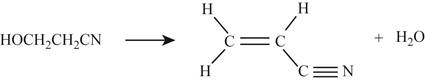
Figure 3
For the reactant,
Hence,
The total energy released when the product is formed
For product,
Hence,
The total energy released when the product is formed
For water,
So, the total energy of products
So the change in energy for the second reaction is,
The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
(b)
Interpretation: The change in energy for the given chemical reactions has to be calculated.
Concept introduction: In a chemical reaction, energy is gained, endothermic reactions, or released, exothermic reactions. The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
To determine: The change in energy for the stated reactions.
Answer to Problem 152CP
The required energy change is
Explanation of Solution
Given
The given reaction is,

Figure 4
For the reactant side,
For
The energy required
For
Total reactant energy
For products,
For
The total energy is
For
Since
For
The total energy for products is
So the change in energy for the second reaction is,
The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
(c)
Interpretation: The change in energy for the given chemical reactions has to be calculated.
Concept introduction: In a chemical reaction, energy is gained, endothermic reactions, or released, exothermic reactions. The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
To determine: The change in energy for the stated reactions.
Answer to Problem 152CP
The required energy change is
Explanation of Solution
Given
For the given reaction,

Figure 5
Energy for reactants,
For
Total energy
(since
For
Total energy
(since
For
The total energy of reactants
Energy for products,
For
The total energy
(since
For
Since
The total energy for products
So the change in energy for the second reaction is,
The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry: An Atoms First Approach
- Provide the structure, circle or draw, of the monomeric unit found in the biological polymeric materials given below. HO OH amylose OH OH 행 3 HO cellulose OH OH OH Ho HOarrow_forwardWhat units (if any) does K have? Does K depend upon how the concentration is expressed (e.g. molarity, ppm, ppb, etc.)? in calculating the response factorarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Don't used Ai solution and don't used hand raitingarrow_forwardOA. For the structure shown, rank the bond lengths (labeled a, b and c) from shortest to longest. Place your answer in the box. Only the answer in the box will be graded. (2 points) H -CH3 THe b Нarrow_forwardDon't used hand raitingarrow_forward
- Quizzes - Gen Organic & Biological Che... ☆ myd21.lcc.edu + O G screenshot on mac - Google Search savings hulu youtube google disney+ HBO zlib Homework Hel...s | bartleby cell bio book Yuzu Reader: Chemistry G periodic table - Google Search b Home | bartleby 0:33:26 remaining CHEM 120 Chapter 5_Quiz 3 Page 1: 1 > 2 > 3 > 6 ¦ 5 > 4 > 7 ¦ 1 1 10 8 ¦ 9 a ¦ -- Quiz Information silicon-27 A doctor gives a patient 0.01 mC i of beta radiation. How many beta particles would the patient receive in I minute? (1 Ci = 3.7 x 10 10 d/s) Question 5 (1 point) Saved Listen 2.22 x 107 222 x 108 3.7 x 108 2.22 x 108 none of the above Question 6 (1 point) Listen The recommended dosage of 1-131 for a test is 4.2 μCi per kg of body mass. How many millicuries should be given to a 55 kg patient? (1 mCi = 1000 μСi)? 230 mCiarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Q3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. CI Cl H3C-Cl CI a) A B C D Br Br b) A B C Br H3C-Br Darrow_forwardQ4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br .OH Brarrow_forwardClassify each molecule as optically active or inactive. Determine the configuration at each H соон Chirality center OH 애 He OH H3C Ноос H H COOH A K B.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





