
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 29, Problem 29.11P
Interpretation Introduction
Interpretation:
A scheme in which maltose is formed, starting with UDP-glucose should be devised.
Concept Introduction:
Phosphorylase enzyme is used to catalyze the glucose molecules in the cell. It results in the formation of glucose unit by cleavage. The equilibrium equation is represented as follows:
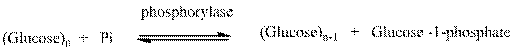
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5. Help
3.Help
4. Help
Chapter 29 Solutions
Introduction to General, Organic and Biochemistry
Ch. 29 - Prob. 29.1PCh. 29 - Prob. 29.2PCh. 29 - Prob. 29.3PCh. 29 - Prob. 29.4PCh. 29 - Prob. 29.5PCh. 29 - Prob. 29.6PCh. 29 - Prob. 29.7PCh. 29 - Prob. 29.8PCh. 29 - Prob. 29.9PCh. 29 - Prob. 29.10P
Ch. 29 - Prob. 29.11PCh. 29 - Prob. 29.12PCh. 29 - Prob. 29.13PCh. 29 - Prob. 29.14PCh. 29 - Prob. 29.15PCh. 29 - Prob. 29.16PCh. 29 - Prob. 29.17PCh. 29 - Prob. 29.18PCh. 29 - Prob. 29.19PCh. 29 - Prob. 29.20PCh. 29 - Prob. 29.21PCh. 29 - Which of these fatty acids can be synthesized by...Ch. 29 - Prob. 29.23PCh. 29 - Prob. 29.24PCh. 29 - Prob. 29.25PCh. 29 - Prob. 29.26PCh. 29 - Prob. 29.27PCh. 29 - Prob. 29.28PCh. 29 - Prob. 29.29PCh. 29 - Prob. 29.30PCh. 29 - Prob. 29.31PCh. 29 - Prob. 29.32PCh. 29 - Prob. 29.33PCh. 29 - Prob. 29.34PCh. 29 - Prob. 29.35PCh. 29 - Prob. 29.36PCh. 29 - Prob. 29.37PCh. 29 - Prob. 29.38PCh. 29 - Prob. 29.39PCh. 29 - Prob. 29.40PCh. 29 - Prob. 29.41PCh. 29 - Prob. 29.42PCh. 29 - Prob. 29.43PCh. 29 - Prob. 29.44PCh. 29 - Prob. 29.45PCh. 29 - Prob. 29.46PCh. 29 - Prob. 29.47PCh. 29 - Prob. 29.48PCh. 29 - Prob. 29.49PCh. 29 - Prob. 29.50PCh. 29 - Prob. 29.51PCh. 29 - Prob. 29.52PCh. 29 - Prob. 29.53PCh. 29 - Prob. 29.54PCh. 29 - Prob. 29.55PCh. 29 - Prob. 29.56PCh. 29 - Prob. 29.57PCh. 29 - Prob. 29.58PCh. 29 - Prob. 29.59PCh. 29 - 29-60 How does the energy source differ in...Ch. 29 - Prob. 29.61PCh. 29 - A vegan diet is one that excludes all animal...Ch. 29 - Prob. 29.63PCh. 29 - Prob. 29.64PCh. 29 - Prob. 29.65PCh. 29 - Prob. 29.66PCh. 29 - Prob. 29.67PCh. 29 - Prob. 29.68P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following are descriptions of possible starting material for this reaction? H ? trace acid an ester a ketone an imine an aldehyde a carboxylic acid an enamine a primary amine a secondary amine a tertiary aminearrow_forwardNonearrow_forwardWhat are the reagents needed for this and the third structure I only got the top right structure rightarrow_forward
- Please label this COZY spectraarrow_forwardPlease label this HNMRarrow_forwardConsider the following gas chromatographs of Compound A, Compound B, and a mixture of Compounds A and B. Inject A B mixture Area= 9 Area = 5 Area = 3 Area Inject . མི། Inject J2 What is the percentage of Compound B in the the mixture?arrow_forward
- Rank these according to stability. CH3 H3C CH3 1 CH3 H3C 1 most stable, 3 least stable O 1 most stable, 2 least stable 2 most stable, 1 least stable O2 most stable, 3 least stable O3 most stable, 2 least stable O3 most stable, 1 least stable CH3 2 CH3 CH3 H₂C CH3 3 CH3 CHarrow_forwardConsider this IR and NMR: INFRARED SPECTRUM TRANSMITTANCE 0.8- 0.6 0.4 0.2 3000 10 9 8 00 HSP-00-541 7 CO 6 2000 Wavenumber (cm-1) сл 5 ppm 4 M Which compound gave rise to these spectra? N 1000 1 0arrow_forwardConsider this reaction (molecular weights are under each compound): HC=CH + 2 HCI --> C2H4Cl 2 MW = 26 36.5 99 If 4.4 g of HC=CH are reacted with 110 mL of a 2.3 M HCI solution, and 6.0 g of product are actually produced, what is the percent yield?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry In Focus
Chemistry
ISBN:9781305084476
Author:Tro, Nivaldo J., Neu, Don.
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co