
Concept explainers
(a)
Interpretation: To indicate whether glutamate is a
Concept introduction: A species containing 4 carbon atoms is said to be a
For example, succinic acid contains 4 carbon atoms and thus it is said to be a
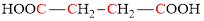
(b)
Interpretation: To indicate whether oxaloacetate is a
Concept introduction: A species containing 4 carbon atoms is said to be a
For example, succinic acid contains 4 carbon atoms and thus it is said to be a
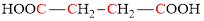
(c)
Interpretation: To indicate whether
Concept introduction: A species containing 4 carbon atoms is said to be a
For example, succinic acid contains 4 carbon atoms and thus it is said to be a
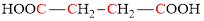
(d)
Interpretation: To indicate whether succinate is a
Concept introduction: A species containing 4 carbon atoms is said to be a
For example, succinic acid contains 4 carbon atoms and thus it is said to be a
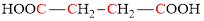
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
General, Organic, and Biological Chemistry
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER




