
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 25.SE, Problem 58AP
Interpretation Introduction
Interpretation:
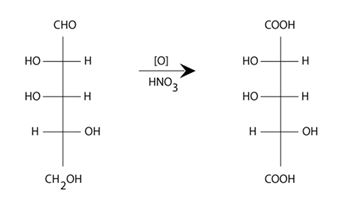
D-lyxose has the Fisher projection formula an [c-pentose] sugar on oxidation with HNO3 the aldaric acid is
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What is the stepwise mechanism for this reaction?
Draw the major product of this reaction
Please provide the IUPAC name for the compound shown here
Chapter 25 Solutions
Organic Chemistry
Ch. 25.1 - Prob. 1PCh. 25.2 - Prob. 2PCh. 25.2 - Prob. 3PCh. 25.2 - Prob. 4PCh. 25.2 - Prob. 5PCh. 25.3 - Prob. 6PCh. 25.3 - Prob. 7PCh. 25.4 - Prob. 8PCh. 25.4 - Prob. 9PCh. 25.4 - Prob. 10P
Ch. 25.5 - Prob. 11PCh. 25.5 - Prob. 12PCh. 25.5 - Prob. 13PCh. 25.5 - Prob. 14PCh. 25.5 - Prob. 15PCh. 25.6 - Prob. 16PCh. 25.6 - Prob. 17PCh. 25.6 - Prob. 18PCh. 25.6 - Prob. 19PCh. 25.6 - Prob. 20PCh. 25.6 - Prob. 21PCh. 25.6 - Prob. 22PCh. 25.6 - Prob. 23PCh. 25.7 - Prob. 24PCh. 25.8 - Show the product you would obtain from the...Ch. 25.SE - Prob. 26VCCh. 25.SE - Prob. 27VCCh. 25.SE - Prob. 28VCCh. 25.SE - Prob. 29VCCh. 25.SE - Prob. 30MPCh. 25.SE - Prob. 31MPCh. 25.SE - Glucosamine, one of the eight essential...Ch. 25.SE - D-Glicose reacts with acetone in the presence of...Ch. 25.SE - Prob. 34MPCh. 25.SE - Prob. 35MPCh. 25.SE - Prob. 36APCh. 25.SE - Prob. 37APCh. 25.SE - Prob. 38APCh. 25.SE - Prob. 39APCh. 25.SE - Prob. 40APCh. 25.SE - Assign R or S configuration to each chirality...Ch. 25.SE - Prob. 42APCh. 25.SE - Prob. 43APCh. 25.SE - Prob. 44APCh. 25.SE - Prob. 45APCh. 25.SE - Prob. 46APCh. 25.SE - Prob. 47APCh. 25.SE - Prob. 48APCh. 25.SE - Prob. 49APCh. 25.SE - Prob. 50APCh. 25.SE - Prob. 51APCh. 25.SE - Prob. 52APCh. 25.SE - Prob. 53APCh. 25.SE - Prob. 54APCh. 25.SE - Prob. 55APCh. 25.SE - Prob. 56APCh. 25.SE - Prob. 57APCh. 25.SE - Prob. 58APCh. 25.SE - Prob. 59APCh. 25.SE - Prob. 60APCh. 25.SE - Prob. 61APCh. 25.SE - Prob. 62APCh. 25.SE - Prob. 63APCh. 25.SE - D-Mannose reacts with acetone to give a...Ch. 25.SE - Prob. 65APCh. 25.SE - Prob. 66APCh. 25.SE - Prob. 67APCh. 25.SE - Prob. 68APCh. 25.SE - Prob. 69APCh. 25.SE - Prob. 70APCh. 25.SE - Prob. 71AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Problem 6-29 Identify the functional groups in the following molecules, and show the polarity of each: (a) CH3CH2C=N CH, CH, COCH (c) CH3CCH2COCH3 NH2 (e) OCH3 (b) (d) O Problem 6-30 Identify the following reactions as additions, eliminations, substitutions, or rearrangements: (a) CH3CH2Br + NaCN CH3CH2CN ( + NaBr) Acid -OH (+ H2O) catalyst (b) + (c) Heat NO2 Light + 02N-NO2 (+ HNO2) (d)arrow_forwardPredict the organic product of Y that is formed in the reaction below, and draw the skeletal ("line") structures of the missing organic product. Please include all steps & drawings & explanations.arrow_forwardPlease choose the best reagents to complete the following reactionarrow_forward
- Problem 6-17 Look at the following energy diagram: Energy Reaction progress (a) Is AG for the reaction positive or negative? Label it on the diagram. (b) How many steps are involved in the reaction? (c) How many transition states are there? Label them on the diagram. Problem 6-19 What is the difference between a transition state and an intermediate? Problem 6-21 Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall AG°, transition states, and intermediate. Is AG° positive or negative? Problem 6-23 Draw an energy diagram for a reaction with Keq = 1. What is the value of AG° in this reaction?arrow_forwardProblem 6-37 Draw the different monochlorinated constitutional isomers you would obtain by the radical chlorination of the following compounds. (b) (c) Problem 6-39 Show the structure of the carbocation that would result when each of the following alkenes reacts with an acid, H+. (a) (b) (c)arrow_forwardPlease draw the major product of this reaction. Ignore inorganic byproducts and the carboxylic side productarrow_forward
- predict the product formed by the reaction of one mole each of cyclohex-2-en-1-one and lithium diethylcuprate. Assume a hydrolysis step follows the additionarrow_forwardPlease handwriting for questions 1 and 3arrow_forwardIs (CH3)3NHBr an acidic or basic salt? What happens when dissolved in aqueous solution? Doesn't it lose a Br-? Does it interact with the water? Please advise.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning