
Concept explainers
a)
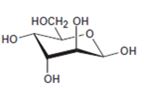
Interpretation:
The open-chain form of the sugar given is to be drawn.
Concept introduction:
The pyranose form is a cyclic hemiacetal form with a six membered ring formed by the nucleophilic addition of the –OH group on C5 to the C1 carbonyl group. The furanose form is a cyclic hemiacetal form with a five membered ring formed by the nucleophilic addition of the –OH group on C5 to the C2 carbonyl group.
The orientation of –OH group differs in α- and β- anomers. In α- anomer the OH on C1 is cis to the –OH at the lowest chirality center in Fischer projection while in β- anomer the –OH on C1 is trans to the –OH at the lowest chirality center in Fischer projection.
D sugars have the –O- at C5 on the right in the uncoiled form while L sugars have -O- at C5 on the left.
To draw:
The open-chain form of the sugar given.
b)
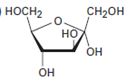
Interpretation:
The open-chain form of the sugar given is to be drawn.
Concept introduction:
The pyranose form is a cyclic hemiacetal form with a six membered ring formed by the nucleophilic addition of the –OH group on C5 to the C1 carbonyl group. The furanose form is a cyclic hemiacetal form with a five membered ring formed by the nucleophilic addition of the –OH group on C5 to the C2 carbonyl group.
The orientation of –OH group differs in α- and β- anomers. In the α- anomer the OH on C1 is cis to the –OH at the lowest chirality center in Fischer projection, while in β- anomer the –OH on C1 is trans to the –OH at the lowest chirality center in Fischer projection.
D sugars have the –O- at C5 on the right in the uncoiled form while L sugars have -O- at C5 on the left.
To draw:
The open-chain form of the sugar given.
c)
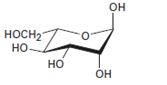
Interpretation:
The open-chain form of the sugar given is to be drawn.
Concept introduction:
The pyranose form is a cyclic hemiacetal form with a six membered ring formed by the nucleophilic addition of the –OH group on C5 to the C1 carbonyl group. The furanose form is a cyclic hemiacetal form with a five membered ring formed by the nucleophilic addition of the –OH group on C5 to the C2 carbonyl group.
The orientation of –OH group differs in α- and β- anomers. In the α- anomer the OH on C1 is cis to the –OH at the lowest chirality center in Fischer projection, while in β- anomer the –OH on C1 is trans to the –OH at the lowest chirality center in Fischer projection.
D sugars have the –O- at C5 on the right in the uncoiled form while L sugars have -O- at C5 on the left.
To draw:
The open-chain form of the sugar given.
Trending nowThis is a popular solution!

Chapter 25 Solutions
Organic Chemistry
- Steps and explanation please. Add how to solve or target similar problems.arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forwardThis organic molecule is dissolved in an acidic aqueous solution: OH OH A short time later sensitive infrared spectroscopy reveals the presence of a new C = O stretch absorption. That is, there must now be a new molecule present with at least one C = O bond. In the drawing area below, show the detailed mechanism that could convert the molecule above into the new molecule. Videos 849 Explanation Check C Click and drag to start dwing a structure. # 3 MAR 23 Add/Remove steparrow_forward||| 7:47 ull 57% ← Problem 19 of 48 Submit Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the product of this carbocation rearrangement. Include all lone pairs and charges as appropriate. H 1,2-alkyl shift +arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forwardBelow is the SN1 reaction of (S)-3-chlorocyclohexene and hydroxide (OH). Draw the missing curved arrows, lone pairs of electrons, and nonzero formal charges. In the third box, draw the two enantiomeric products that will be produced. 5th attempt Please draw all four bonds at chiral centers. Draw the two enantiomeric products that will be produced. Draw in any hydrogen at chiral centers. 1000 4th attempt Feedback Please draw all four bonds at chiral centers. 8. R5 HO: See Periodic Table See Hint H Cl Br Jid See Periodic Table See Hintarrow_forwardShow that a molecule with configuration π4 has a cylindrically symmetric electron distribution. Hint: Let the π orbitals be equal to xf and yf, where f is a function that depends only on the distance from the internuclear axis.arrow_forward(a) Verify that the lattice energies of the alkali metal iodides are inversely proportional to the distances between the ions in MI (M = alkali metal) by plotting the lattice energies given below against the internuclear distances dMI. Is the correlation good? Would a better fit be obtained by plotting the lattice energies as a function of (1 — d*/d)/d, as theoretically suggested, with d* = 34.5 pm? You must use a standard graphing program to plot the graph. It generates an equation for the line and calculates a correlation coefficient. (b) From the graph obtained in (a), estimate the lattice energy of silver iodide. (c) Compare the results of (b) with the experimental value of 886 kJ/mol. If they do not agree, explain the deviation.arrow_forwardCan I please get help with #3 & 4? Thanks you so much!arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios

 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





