
Interpretation:
A structure for atropine (C17H23NO3) is to be proposed from the following observations: i) On basic hydrolysis atropine yields tropic acid C6H5CH(CH2OH)COOH and tropine (C8H15NO). ii) Tropine is an optically inactive alcohol that yields tropidene on dehydration with H2SO4.
Concept introduction:
Esters when hydrolyzed with bases yield alcohols and
To predict:
A structure for atropine (C17H23NO3) from the following observations: i) On basic hydrolysis atropine yields tropic acid C6H5CH(CH2OH)COOH and tropine (C8H15NO). ii) Tropine is an optically inactive alcohol that yields tropidene on dehydration with H2SO4.
Answer:
Structure of atropine is
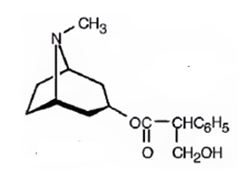
Explanation:
Atropine gives tropic acid and an alcohol, tropine when hydrolyzed in the presence of bases. Hence it must be an ester. Tropine upon dehydration gives tropidene. Hence structure of tropine (optically inactive) can be obtained by adding water to the structure of tropidene.
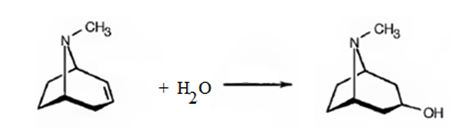
The formation of the ester linkage between the OH of atropine and COOH of tropic acid gives atropine. The reactions can be represented as below.
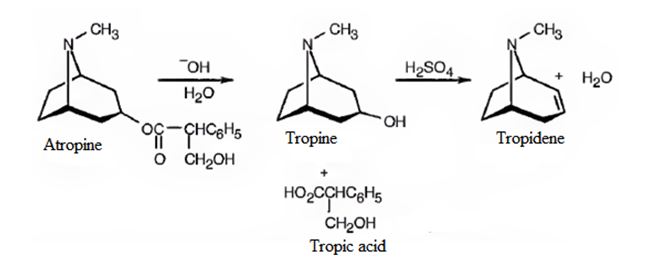
Conclusion:
Structure of atropine is

Trending nowThis is a popular solution!

Chapter 24 Solutions
Organic Chemistry
- Complete the following esterification reaction by drawing the structural formula of the product formed. HOH HO i catalyst catalyst OH HO (product has rum flavor) (product has orange flavor)arrow_forwardThe statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG stands for the standard Gibbs free energy of reaction and K stands for the equilibrium constant. In each table, there may be one statement that is faise because it contradicts the other three statements. If you find a false statement, check the box next to t Otherwise, check the "no false statements" box under the table. statement false? AG"1 no false statements: statement false? AG-0 0 InK-0 0 K-1 0 AH-TAS no false statements 2arrow_forwardComplete the following esterification reactions by drawing the line formulas of the carboxylic acid and alcohol required to form the ester shown. catalyst catalyst catalyst apricot fragrancearrow_forward
- Show the saponification products of the following ester: You don't need to draw in the Na+ cation. catalyst, A catalyst, A catalyst, Aarrow_forwardWhat would happen if the carboxylic acid and alcohol groups were on the same molecule? In essence, the molecule reacts with itself. Draw the structure of the products formed in this manner using the reactants below. If two functional groups interact with one another on the same molecule, this is called an “intramolecular" (within one) rather than "intermolecular" (between two or more) attack. OH OH catalyst OH HO catalyst catalyst HO OHarrow_forwardQ3: Write in the starting alkyl bromide used to form the following products. Include any reactants, reagents, and solvents over the reaction arrow. If more than one step is required, denote separate steps by using 1), 2), 3), etc. H OH racemic OH OH 5 racemicarrow_forward
- Draw the Lewis structure of the SO3-O(CH3)2 complex shown in the bottom right of slide 2in lecture 3-3 (“Me” means a CH3 group) – include all valence electron pairs and formal charges.From this structure, should the complex be a stable molecule? Explain.arrow_forwardPredict all organic product(s), including stereoisomers when applicable.arrow_forwardQ5: Propose a reasonable synthesis for the following decalin derivative. using only decalin and alkanes of 3 or fewer carbons. Decalin H3C HO க CH3arrow_forward
- 2Helparrow_forwardplease add appropriate arrows, and tell me clearly where to add arrows, or draw itarrow_forwardWhat I Have Learned Directions: Given the following reaction and the stress applied in each reaction, answer the question below. A. H2(g) + Cl2(g) 2 HCl(g) Stress applied: Decreasing the pressure 1. What is the Keq expression? 2. What will be the effect in the number of moles of HCl(g)? 3. What will be the Equilibrium Shift or the reaction? B. Fe3O4(s) + 4 H2(g) + heat 53 Fe(s) + 4 H₂O(g) Stress applied: Increasing the temperature 1. What is the Keq expression?. 2. What will be the effect in the volume of water vapor collected? 3. What will be the Equilibrium Shift or the reaction? C. 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) + heat Stress applied: Increasing the volume of the container 1. What is the Keq expression?. 2. What will be the effect in the amount of H₂O? 3. What will be the Equilibrium Shift or the reaction?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning



