
Interpretation:
The percentages of neutral and protonated forms present in a solution of 0.0010M pyrimidine at pH = 7.3 are to be calculated if the pKa of pyrimidinium ion is 1.3.
Concept introduction:
pH And pKa are related by Henderson-Hasselbalch equation as
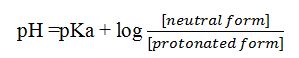
Knowing pH and pKa values, the ratio between the two forms and from which their percentages can be determined.
To calculate:
The percentages of neutral and protonated forms present in a solution of 0.0010M pyrimidine at pH = 7.3, if the pKa of pyrimidinium ion is 1.3.
Answer:
At pH = 7.3, almost 100% pyrimidine molecules exist in the neutral form.
Explanation:
Deprotonation of the ammonium ion of a base can be represented as,

The Henderson-Hasselbalch can be written as
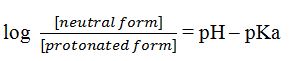
Substituting the values of pH and pKa, we get

Thus the concentration of neutral form is 106 times more than the protonated form. Hence almost 100% pyrimidine molecules exist in the neutral form.
Conclusion:
At pH = 7.3, almost 100% pyrimidine molecules exist in the neutral form.
Trending nowThis is a popular solution!

Chapter 24 Solutions
Organic Chemistry
- Fill-in-the molecules for the oxidation or reduction of the starting alcohol.arrow_forwardName the following carbohydrates give both the systematic and common names. Don't forget to identify the Isomer.arrow_forwardWhat is the product of the reaction of XeF4 with H2O? Group of answer choices H2XeF2 H2XeF4 XeO3 H2XeOarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax


