
Concept explainers
a) CH3CHNH2 or CH3CH2 CONH2
Interpretation:
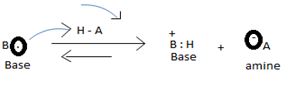
The levis concept (due to GN levis) which is the most general concept of a base defines a base as any species which is sufficient electron rich and contains at last one or more unshared electron pains available for donation, which subsequent electronic interaction or an apparent band formation with an acid, an electron deficient species.
Answer:
Ethylamine CH3CH2NH2 is more basic the amide (ethylamide) CH3CH2CoNH2.
Explanation:
Thus basicity is an attribute defecting the extent of overlapping of this done electron pain donation. Quantitative it is measured by this extent; and also, any structural or chemical environmental factor that tends to increase or active increase the extent of available of donation of this electron pain(s) to a nembutal acid is acid to enhance the basicity of a levis base.
On the basis of the above, we examine what structure or other factor in the two given molecules amide and amine lead then to the bases at all in the fast; and what facter causes them to be which laser copying strengths.
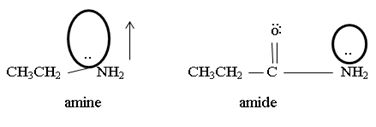
1) Fact I, Both are nitrogenous bases ie the basicity is due, intermediate to the problem of an unshared electron pair on the nitrogen atom of the –NH2-amine group. Thus more available then electron pair of on incident acid, the stronger is the base. Closely, the base strength all the diminished of any factor causes this electron pair to the less available for be genetics within appropriate acid.
2) Fact II, → considered ethylamine and ethanamide.
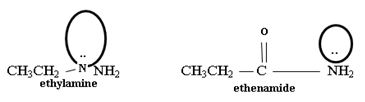
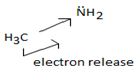
Ethanamide is much stronger base because of the electron releasing positive inductive +g effect of the ethyl (alkyl) group makes the N lone pair more available to any incident proton H+.
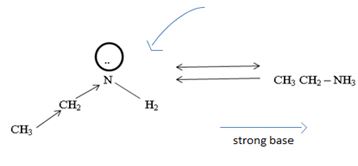
Havens is ethanamide, true is an incident stability caused by delocalization of the nitrogen line-pair electron through orbital overall with the carbonyl group. In resonance terms, amides are more states, and also then reactive than amides because this are hybrids of two resonance forms this amide resonance stabilization is lost when the nitrogen atom is protonated. So petroleum is disfavored. The following structural resonance form and electron density diagrams shown a significants reduced electron density on the amide nitrogen.
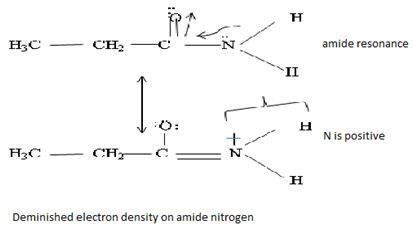
Oxygen – pauling electrons acids = 3.44
Nitrogen – pauling electrons acids = 3.04
Further the very electrons acid values of N is O, specifies the electron delocalization form N to O in amides, and consolidate the structure resonance forms.
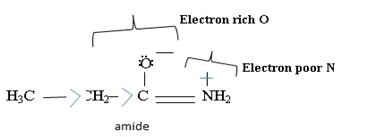
Thus, the very factor that lands amides then reactive also causes their reduced basicity than
Conclusion:
Form the above teach of relative basicity, it comes urtherit saying that population bile basics acids are governed by structural features in substances and chemical environments.
b) NCOH or CH3NH2
Interpretation:
As the outset, let it be said that acidity and basicity are measures of equilibrium (
Sodium hyduxide is a potentates much stronger base than methylamine.
Explanation:
The stronger this officials the more is the ease of proton uptake, and more is the hold on this proton; thus the stronger the species as a base.
Organic bases are potencies much stronger bases than organic bases, v3 amines. Thus NCOH is a much stronger base then CH3NH2 an methylamine CH3NH2, the basicity is attributed to the presence of the unshared electron paid on the nitrogen of the amino group. The extent of available of this electron pair is increased by the inductive release of electron dentist by the methyl group. This miles methylamine strong organic base, but ten, then NAOH, the inorganic base.
Conclusion:
Sodium hyduxide is a potentates much stronger base than methylamine.
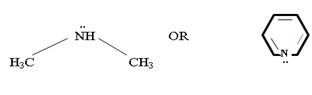
c) CH3NHCH3 or pyridine
Interpretation:
In organic bases, the basics is exhibited to the extent of available of a lone pair(s) of electron an nitrogen atom of the amino group, the more available then electron pair for uptake of a proton, the stronger the base.
Explanation:
In dimethyl amine, inductive release of electrons by the two methyl groups increase the electron density on the nitrogen atom to an appropriate degree. The compound is thus a very strong base, convenes, in the heterocyclic amine pyridine, the line electron pair on nitrogen is lost by amides delocalization into the benzene ring and nitrogen causes c positive change. Thus it is a very week base.
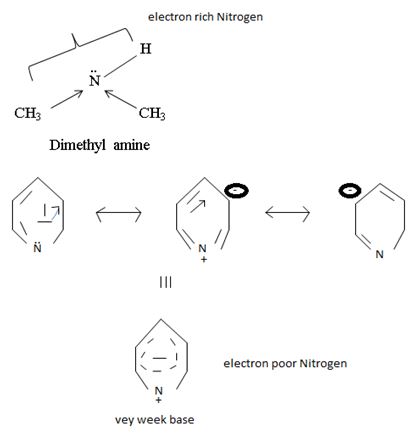
Conclusion:
Basicity in organic amines thus is a measure of the extent of availability of an unshared electron pair on the amines stronger amines than both
Trending nowThis is a popular solution!
Learn your wayIncludes step-by-step video

Chapter 24 Solutions
Organic Chemistry
- The aim of the lab is to measure the sodium content from tomato sauce using the Mohr titration method. There are two groups being: Regular Tomato sauce & Salt Reduced tomato sauce QUESTION: State how you would prepare both Regular & Salt reduced tomato sauce samples for chemical analysis using the Mohr titration methodarrow_forwardUsing the conditions of spontaneity to deduce the signs of AH and AS Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds faster at temperatures above -48. °C. ΔΗ is (pick one) ✓ AS is (pick one) B This reaction is spontaneous except below 114. °C but proceeds at a slower rate below 135. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is C This reaction is exothermic and proceeds faster at temperatures above -43. °C. (pick one) AS is (pick one) v Х 5 ? 18 Ararrow_forwardion. A student proposes the following Lewis structure for the perchlorate (CIO) io : :0: : Cl : - - : :0: ك Assign a formal charge to each atom in the student's Lewis structure. atom central O formal charge ☐ top O ☐ right O ☐ bottom O ☐ Cl ☐arrow_forward
- Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure Yes. Is the proposed Lewis structure reasonable? Cl- : 2: :Z: :Z: N—N : 0: C C1: O CO No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0". ☑arrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions ΔΗ is (pick one) A This reaction is faster above 103. °C than below. AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous only above -9. °C. AS is (pick one) ΔΗ is (pick one) C The reverse of this reaction is always spontaneous. AS is (pick one) 18 Ararrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds slower at temperatures below 41. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 94. °C. AS is (pick one) This reaction is always spontaneous, but ΔΗ is (pick one) C proceeds slower at temperatures below −14. °C. AS is (pick one) Х 00. 18 Ar 무ㅎ B 1 1arrow_forward
- Draw the product of the reaction shown below. Ignore inorganic byproducts. + H CH3CH2OH HCI Drawingarrow_forwardplease explain this in simple termsarrow_forwardK Most Reactive Na (3 pts) Can the metal activity series (shown on the right) or a standard reduction potential table explain why potassium metal can be prepared from the reaction of molten KCI and Na metal but sodium metal is not prepared from the reaction of molten NaCl and K metal? Show how (not). Ca Mg Al с Zn Fe Sn Pb H Cu Ag Au Least Reactivearrow_forward
- (2 pts) Why is O2 more stable as a diatomic molecule than S2?arrow_forwardDraw the Lewis structure for the polyatomic phosphite (PO¾³¯) a anion. Be sure to include all resonance structures that satisfy the octet rule. C I A [ ]¯arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. :0: Cl C C1: 0=0: : 0 : : 0 : H C N No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. ☐ No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".arrow_forward
