
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 24.SE, Problem 41MP
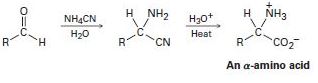
α-Amino acids can be prepared by the Strecker synthesis, a two-step process in which an
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).
What is the IUPAC name of the following compound?
CH₂CH₂
H
CI
H₂CH₂C
H
CH₂
Selected Answer:
O
(35,4R)-4 chloro-3-ethylpentane
Correct
Chapter 24 Solutions
Organic Chemistry
Ch. 24.1 - Name the following compounds:Ch. 24.1 - Prob. 2PCh. 24.1 - Prob. 3PCh. 24.3 - Prob. 4PCh. 24.3 - The benzylammonium ion (C6H5CH2NH3+) has pKa =...Ch. 24.4 - Prob. 6PCh. 24.5 - Prob. 7PCh. 24.6 - Prob. 8PCh. 24.6 - Prob. 9PCh. 24.6 - Show two methods for the synthesis of dopamine, a...
Ch. 24.6 - Prob. 11PCh. 24.6 - Prob. 12PCh. 24.6 - Prob. 13PCh. 24.7 - Prob. 14PCh. 24.7 - Prob. 15PCh. 24.8 - Prob. 16PCh. 24.8 - Propose syntheses of the following compounds from...Ch. 24.8 - How would you prepare the following compounds from...Ch. 24.8 - Prob. 19PCh. 24.9 - Draw an orbital picture of thiazole. Assume that...Ch. 24.9 - Prob. 21PCh. 24.9 - Prob. 22PCh. 24.9 - Which nitrogen atom in the hallucinogenic indole...Ch. 24.9 - Prob. 24PCh. 24.10 - Prob. 25PCh. 24.SE - Name the following amines, and identify each as...Ch. 24.SE - The following compound contains three nitrogen...Ch. 24.SE - Name the following amine, including R, S...Ch. 24.SE - Prob. 29VCCh. 24.SE - Predict the product(s) for each reaction below and...Ch. 24.SE - Predict the product(s) and provide the complete...Ch. 24.SE - Prob. 32MPCh. 24.SE - Predict the product(s) and provide the mechanism...Ch. 24.SE - The diazotization of aniline first involves the...Ch. 24.SE - Prob. 35MPCh. 24.SE - Prob. 36MPCh. 24.SE - Prob. 37MPCh. 24.SE - Prob. 38MPCh. 24.SE - Choline, a component of the phospholipids in cell...Ch. 24.SE - Prob. 40MPCh. 24.SE - -Amino acids can be prepared by the Strecker...Ch. 24.SE - Prob. 42MPCh. 24.SE - Prob. 43MPCh. 24.SE - Prob. 44MPCh. 24.SE - Propose a mechanism for the following reaction:Ch. 24.SE - Prob. 46MPCh. 24.SE - Name the following compounds:Ch. 24.SE - Prob. 48APCh. 24.SE - Prob. 49APCh. 24.SE - Prob. 50APCh. 24.SE - Histamine, whose release in the body triggers...Ch. 24.SE - Prob. 52APCh. 24.SE - Prob. 53APCh. 24.SE - Prob. 54APCh. 24.SE - Prob. 55APCh. 24.SE - Prob. 56APCh. 24.SE - Prob. 57APCh. 24.SE - Prob. 58APCh. 24.SE - Prob. 59APCh. 24.SE - Prob. 60APCh. 24.SE - Show the products from reaction of p-bromoaniline...Ch. 24.SE - Prob. 62APCh. 24.SE - How would you prepare the following compounds from...Ch. 24.SE - Prob. 64APCh. 24.SE - Phenacetin, a substance formerly used in...Ch. 24.SE - Prob. 66APCh. 24.SE - Draw the structure of the amine that produced the...Ch. 24.SE - Fill in the missing reagents a-e in the following...Ch. 24.SE - Prob. 69APCh. 24.SE - Prob. 70APCh. 24.SE - Deduce the structure of the compound with formula...Ch. 24.SE - Prob. 72APCh. 24.SE - Prob. 73APCh. 24.SE - Prob. 74APCh. 24.SE - Prob. 75APCh. 24.SE - Prob. 76APCh. 24.SE - Propose a structure for the product with formula...Ch. 24.SE - Prob. 78APCh. 24.SE - Prob. 79APCh. 24.SE - Prob. 80APCh. 24.SE - Prob. 81APCh. 24.SE - Prob. 82APCh. 24.SE - Prob. 83APCh. 24.SE - Prob. 84AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forwardLook at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forward
- Given 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- Concentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forwardExplain why the following names of the structures are incorrect. CH2CH3 CH3-C=CH-CH2-CH3 a. 2-ethyl-2-pentene CH3 | CH3-CH-CH2-CH=CH2 b. 2-methyl-4-pentenearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License